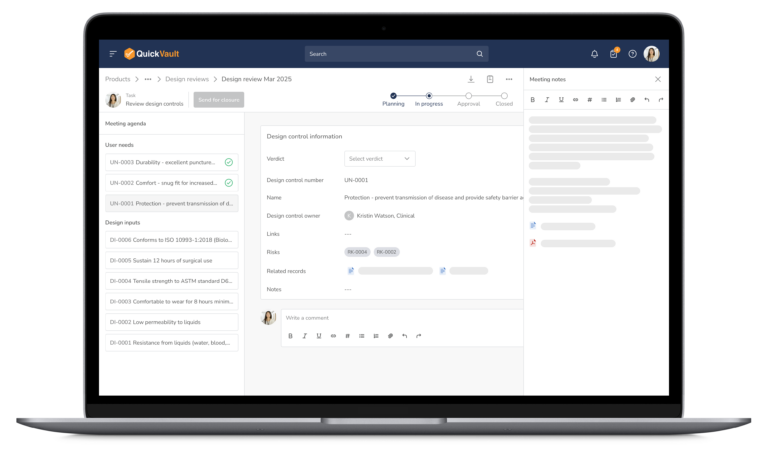
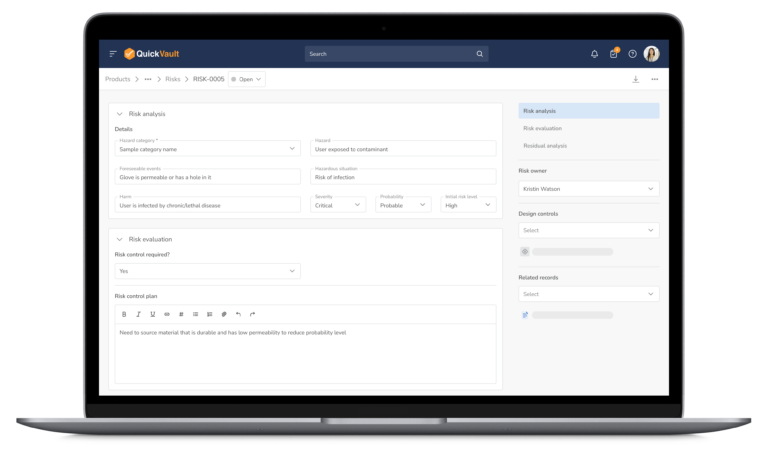
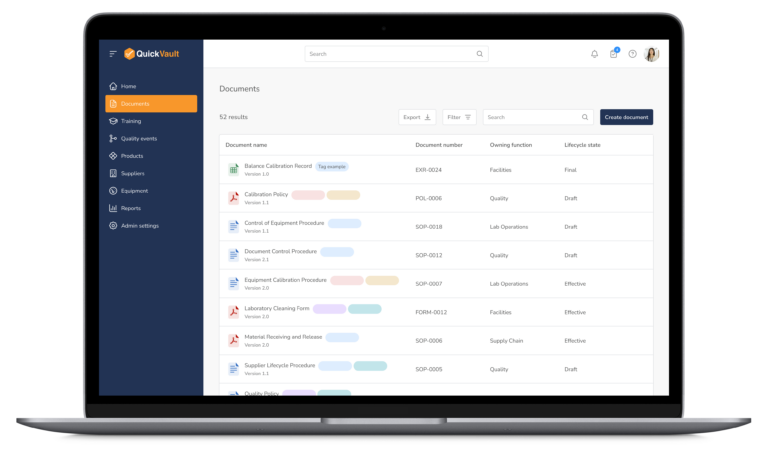
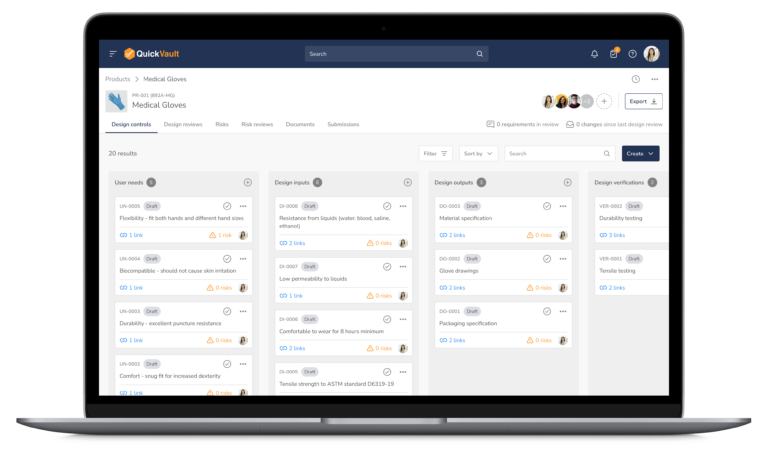
Audit-Ready Software for MedTech Compliance
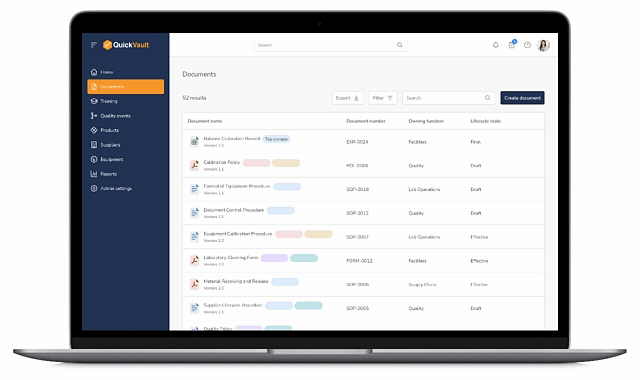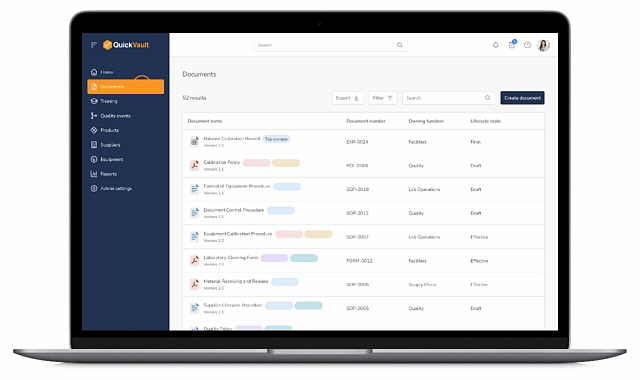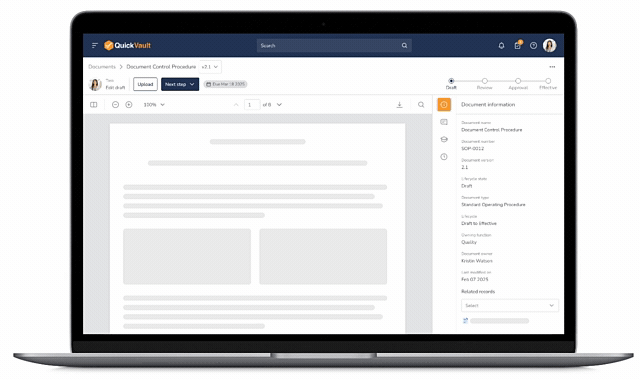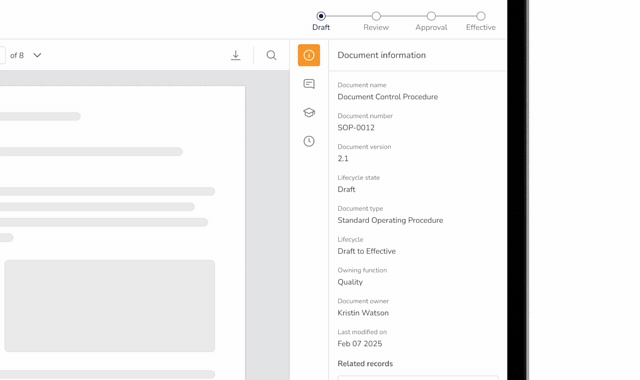
Pass Every Audit with Confidence
Audits are intimidating and consume vast resources to get ready for. Stay prepared and in control with QuickVault.