The All-in-One Platform
to Get Your Medical Device to Market
QuickVault unifies product development, regulatory compliance, quality management, and operations in a single, purpose-built cloud solution for MedTech teams.
A Unified Solution
MedTech success doesn’t stop at regulatory approval.
QuickVault is designed to support your entire product lifecycle, ensuring efficiency, quality, and compliance at every stage of your journey.
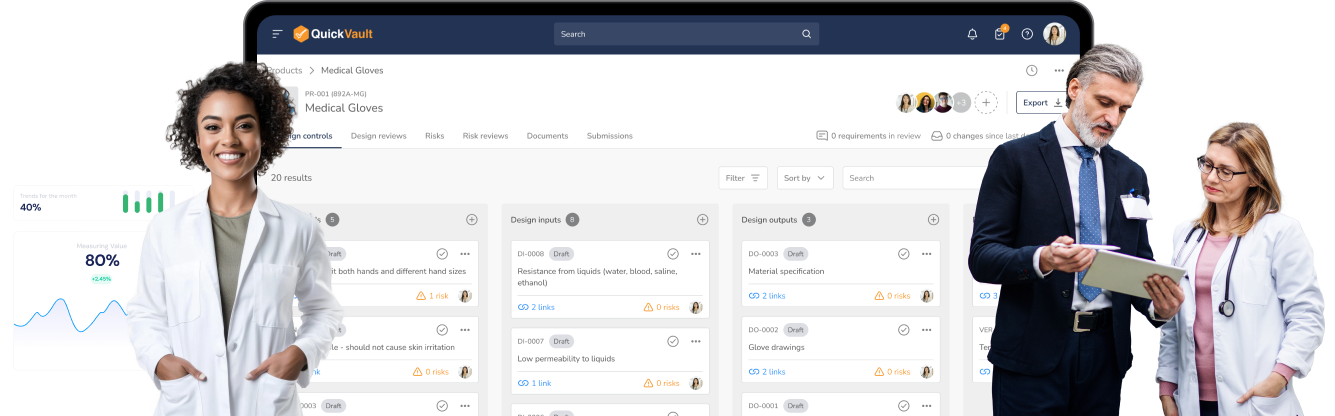
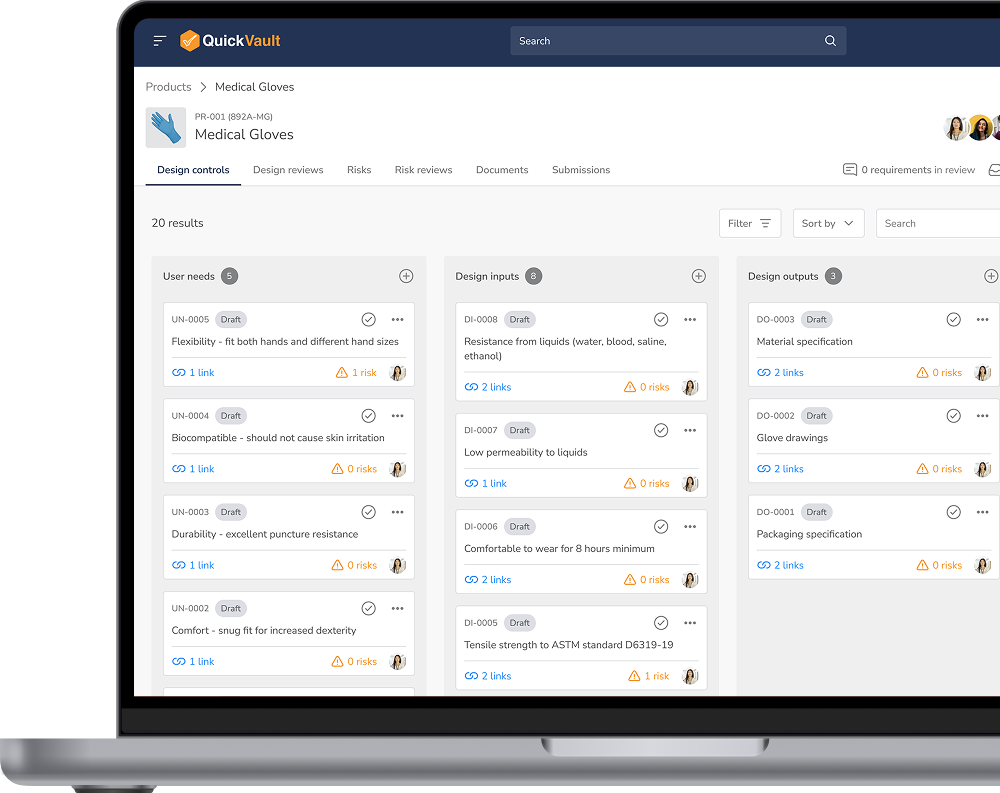
Manage your design controls, design reviews, and risk assessments in a fully compliant, validated environment.
Track every decision, collaborate seamlessly, and ensure you’re developing a safe product that meets regulatory standards from the start.
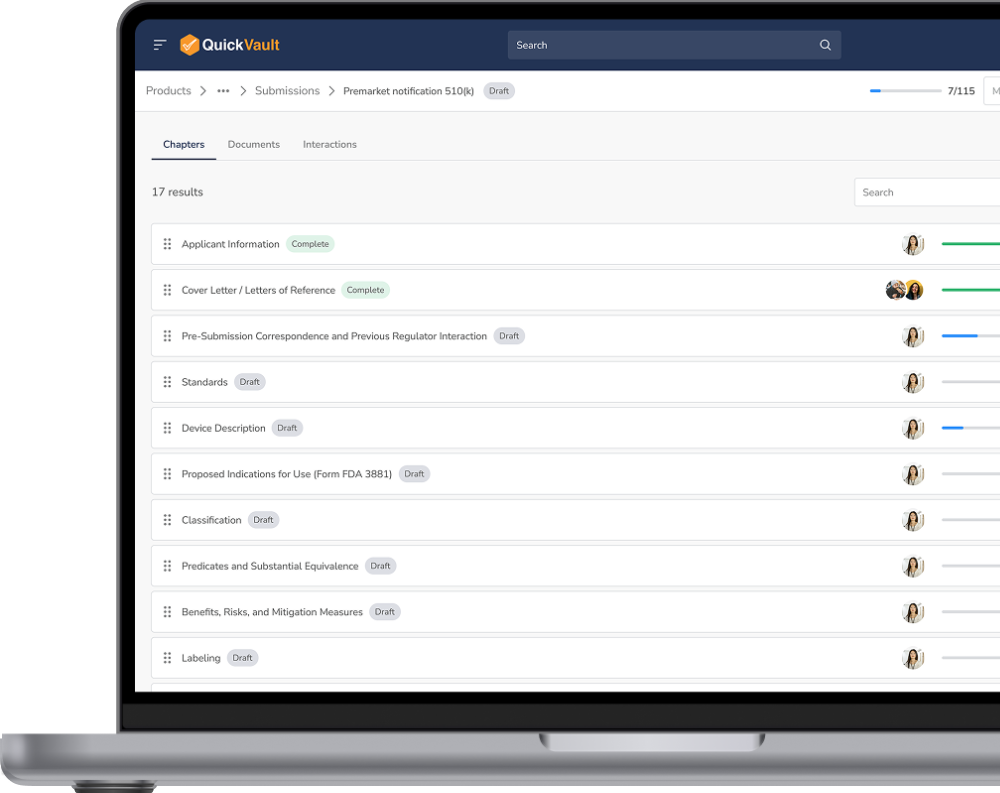
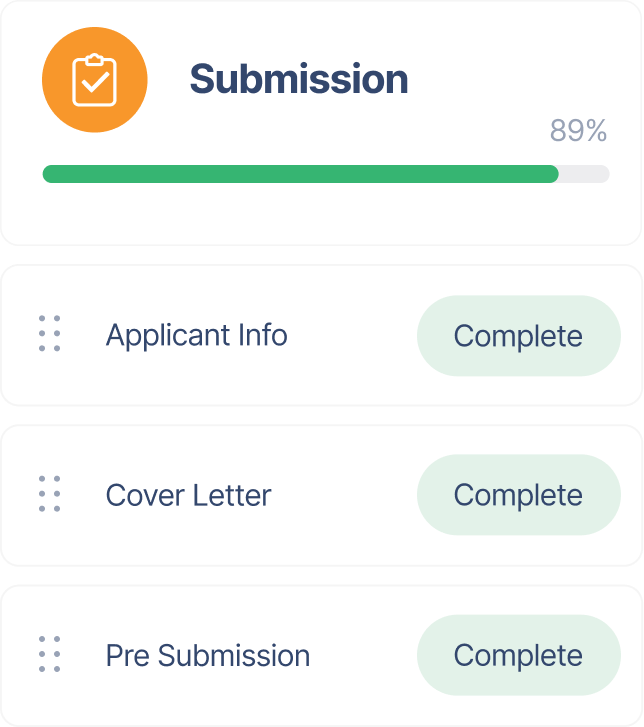
Streamline your product submission, regulatory interactions, and product registration with built-in compliance workflows.
Keep all documentation organized, track submissions in real-time, and respond to regulatory inquiries with ease.
From document control to training and supplier management, internal and external stakeholders can collaborate in a secure, easy-to-use platform.
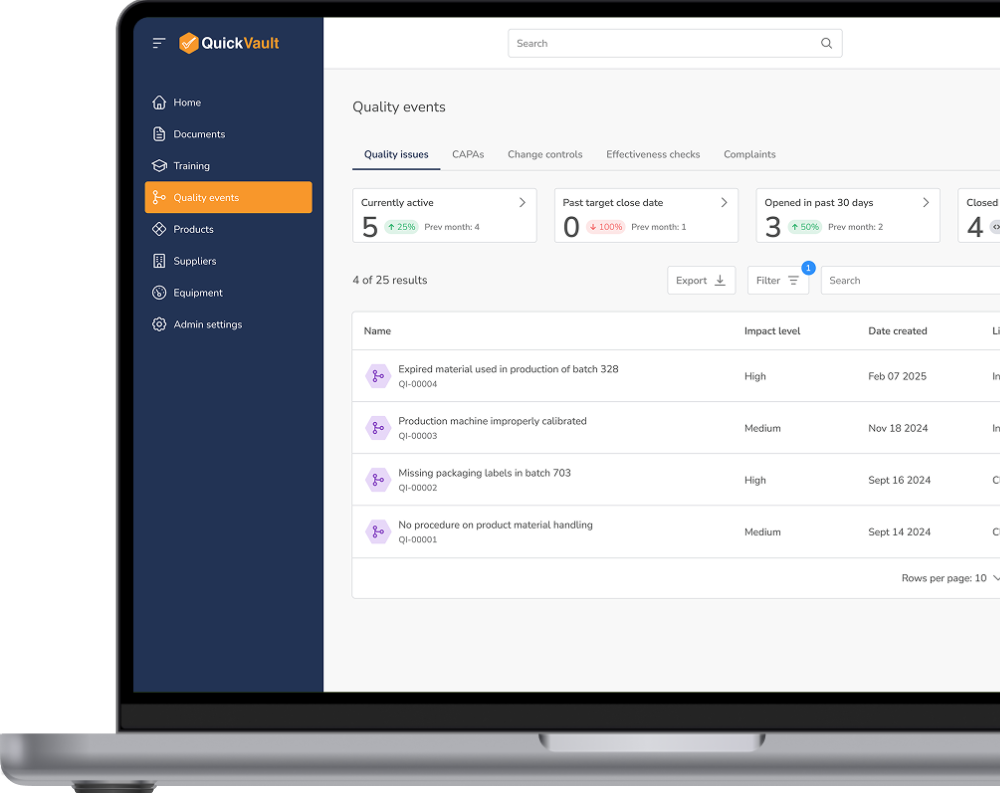
Maintain full visibility into quality processes, automate compliance tracking, and mitigate risks before they become costly issues.
The Device-To-Market Platform for MedTech
Bringing a medical device to market is just the beginning. True success requires a seamless approach to design, regulatory submission, and ongoing production—all while maintaining compliance and quality at every stage.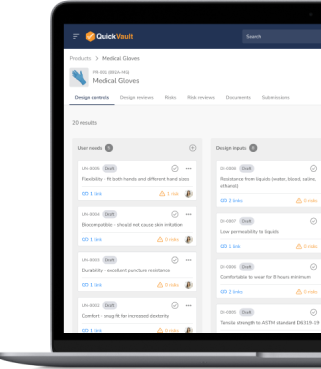
Compliance Without Complexity
Fully validated and compliant with 21 CFR Part 11 / EU Annex 11.

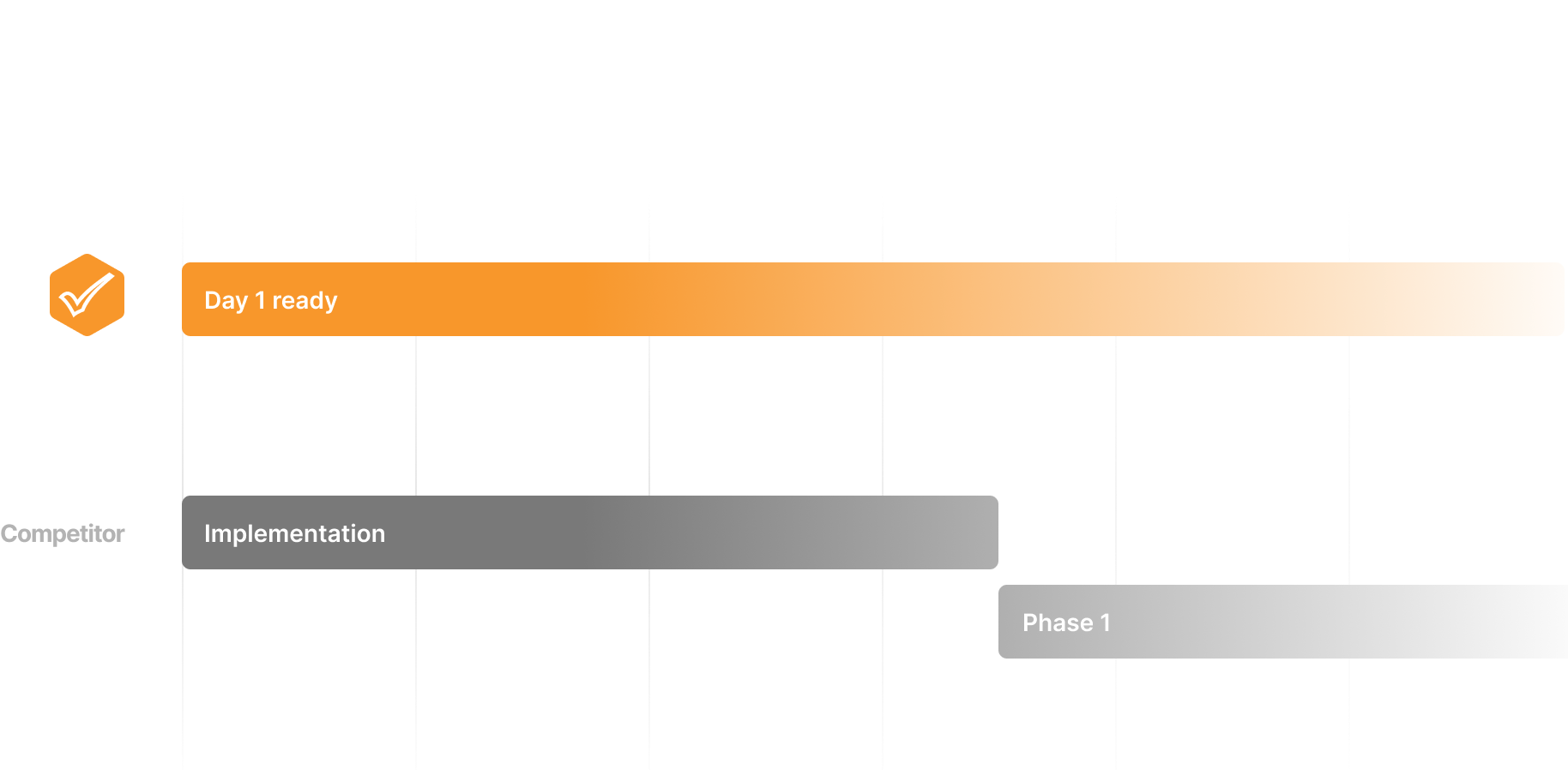
Day 1 Ready
No lengthy implementations or customization projects. Get up and running immediately.
Purpose Built for MedTech
Designed specifically for small and emerging medical device companies, scaling with you as your product and business grow.

All-in-One Platform
Intuitive software that integrates design, regulatory submission, and production into a unified, easy-to-use platform.
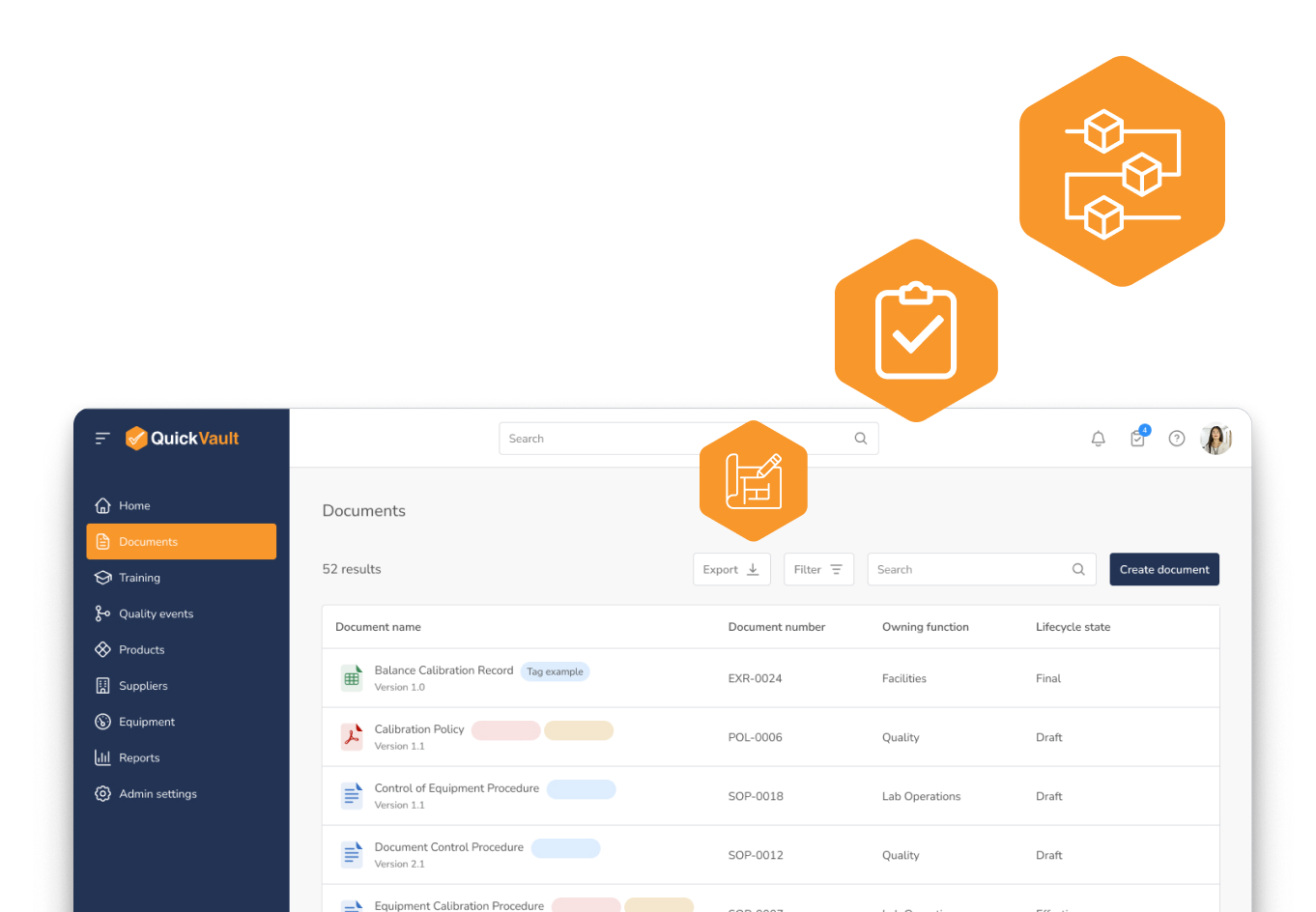
Quality Isn’t Overhead. It’s a Competitive Advantage.
See how a strong, audit-ready QMS accelerates innovation, shortens time to market, and builds investor confidence for MedTech teams.
Our Solutions
Compliance Without Compromise.
QuickVault helps early stage MedTech companies bring devices to market and keep them there by providing the right tools at every stage of growth.
Whether you’re looking for a formal design control system, preparing for a product submission, or managing post-market activities, QuickVault is built to scale with you.
Explore Our SolutionsWe’re A Trusted Authority in Medtech
140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

140+
Medtech Customers

#2 Future 50 Company

#8 Fastest Growing Public Tech Company

WEBINAR RECAP

Mastering Equipment Qualification & Process Validation in MedTech Development
Reliable medical device manufacturing begins long before production: it starts with properly qualified equipment and validated processes. Without these foundations, even the best designs risk inconsistency, delays, and costly rework.
In this free webinar, we’ll walk through the core principles and best practices behind equipment qualification and process validation, including real-world insights into how these activities ensure reproducibility, reduce variability, and build confidence in your manufacturing process. Watch the on-demand recording and get access to free resources now!
Watch On-Demand