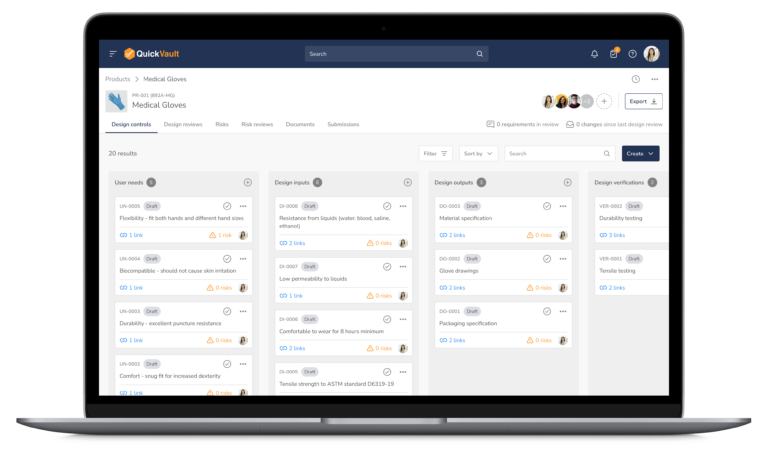
Regulatory Submission Management Software for MedTech
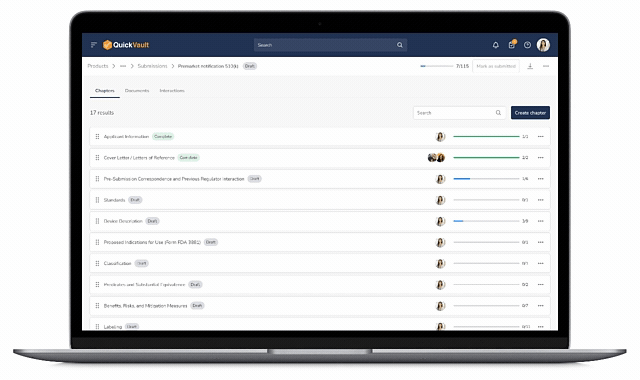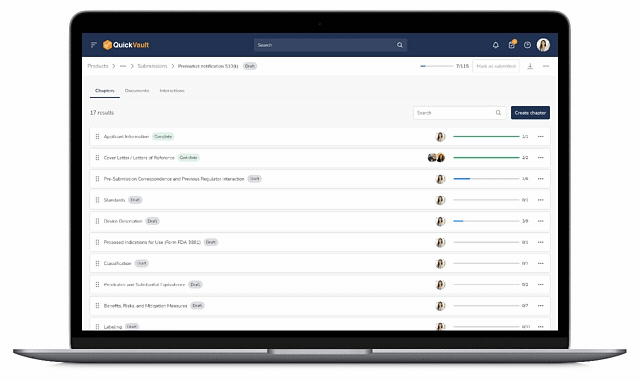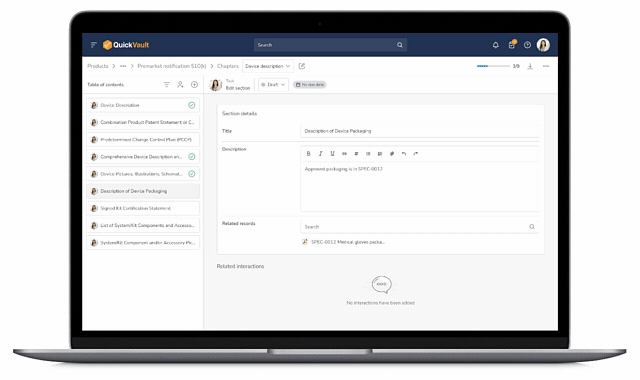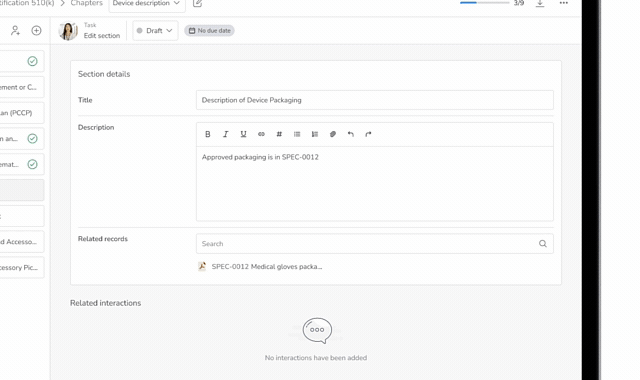
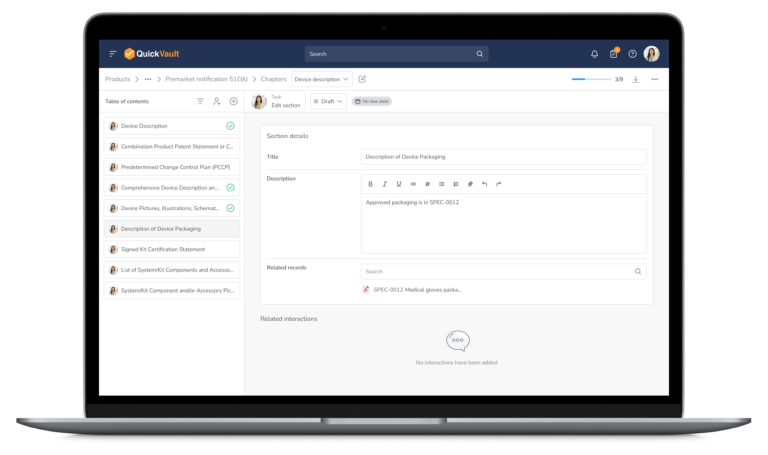
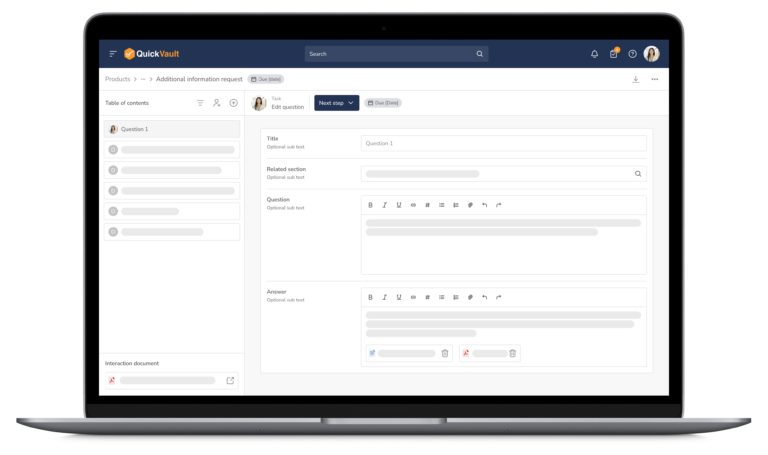
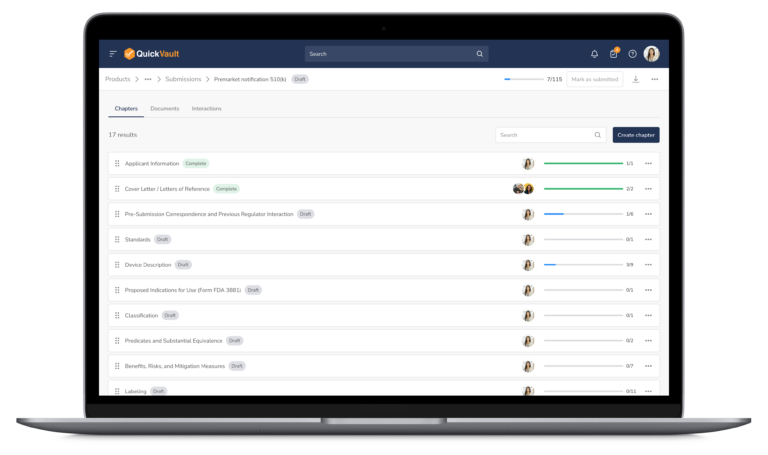
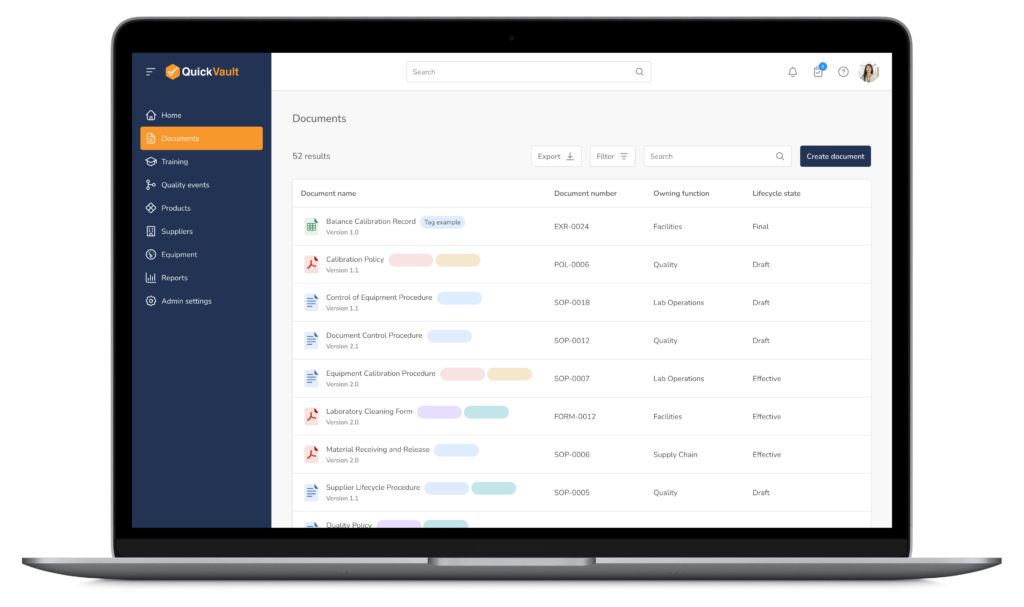
Accelerate Regulatory Submissions from Planning to Approval
Effectively manage every part of your submission process, from initial planning to final clearance or approval in one connected platform.