Device-to-Market Platform For MedTech Success
Designed for MedTech Success. Built for Speed.
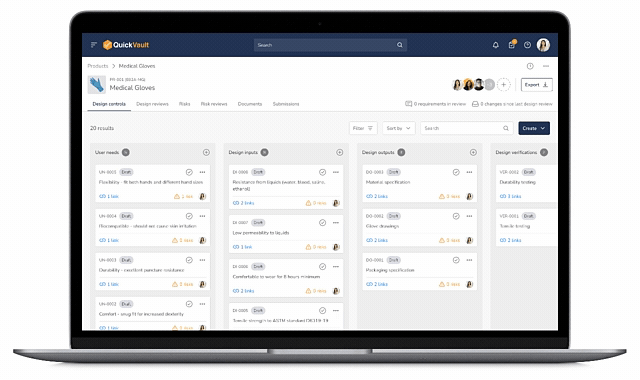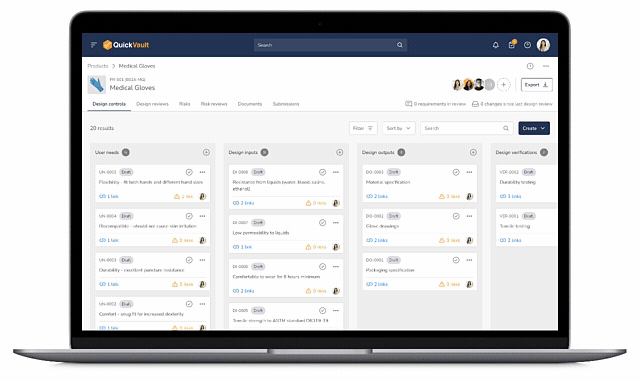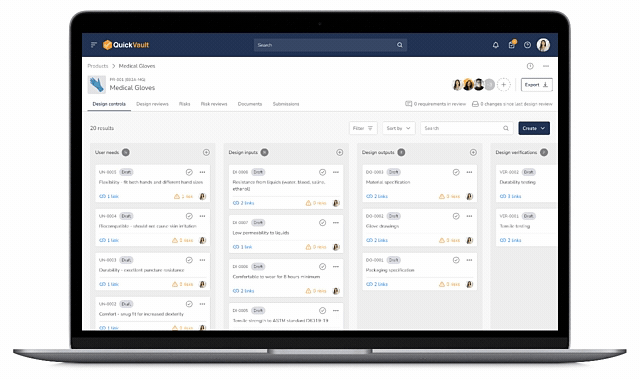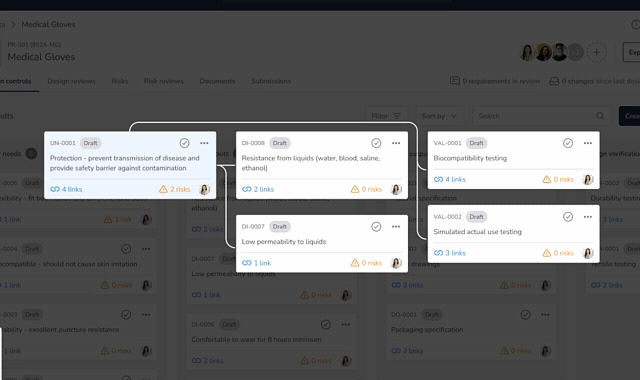
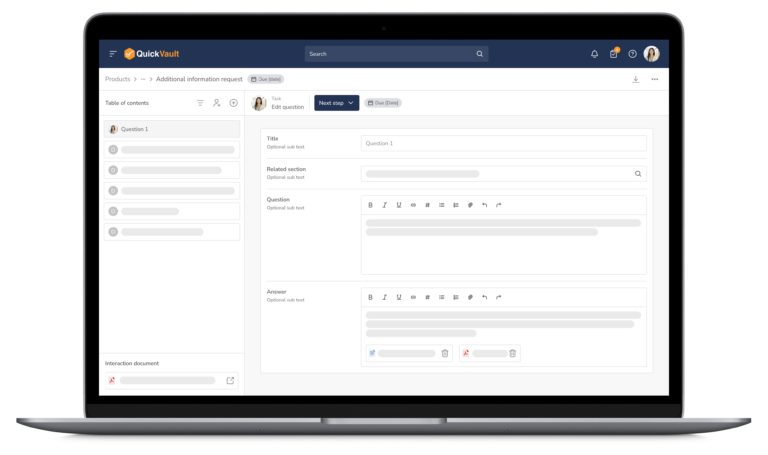
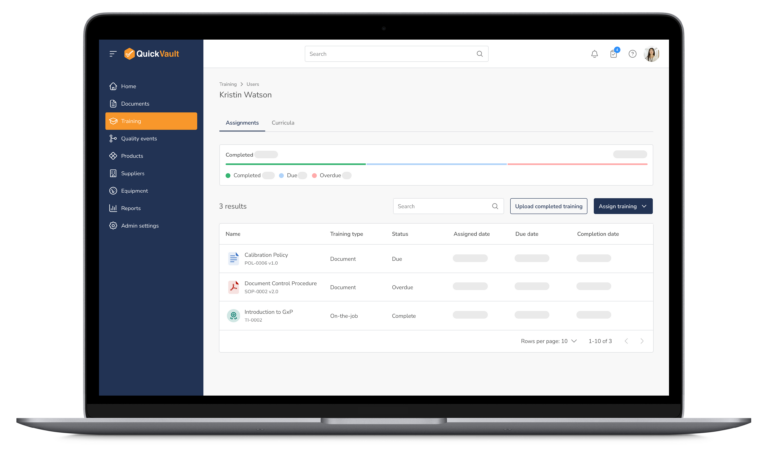
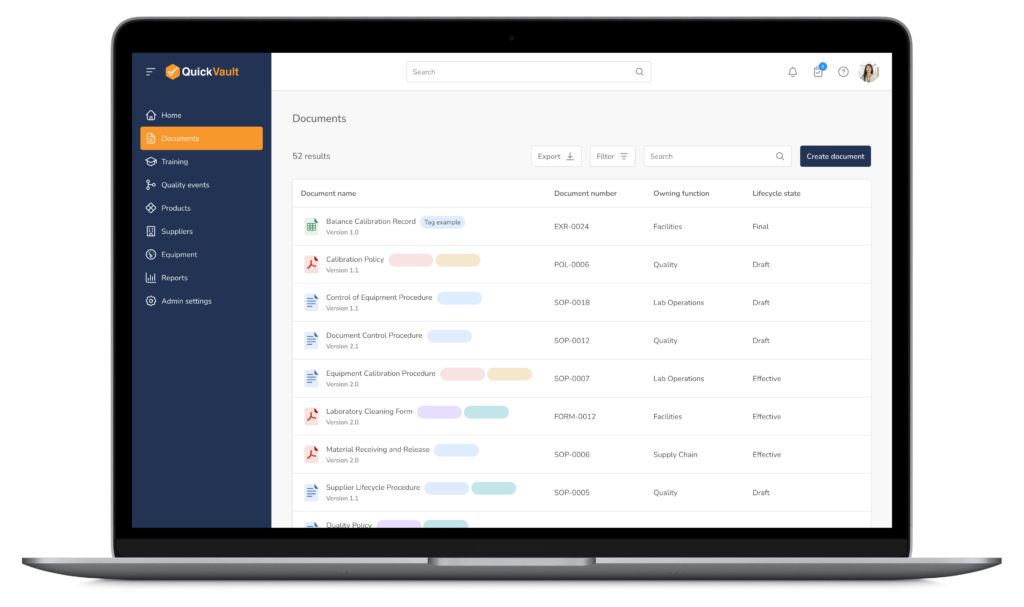
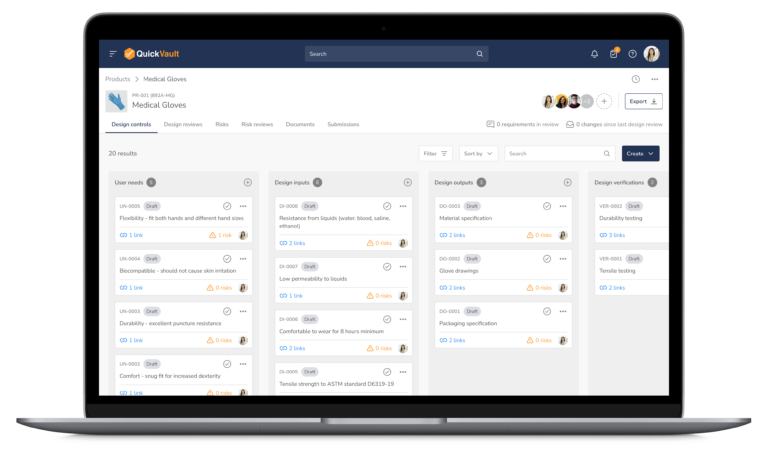
Bringing a medical device to market and scaling your business is complex. QuickVault simplifies this process, freeing you to focus on innovation while ensuring compliance, successful regulatory submissions, and robust infrastructure for commercial growth.