Medical Device Supplier Management Software
Take Control of Supplier Management in One Connected System
Approve, manage and connect suppliers to products, documents, equipment, and quality events for complete visibility across your supply chain.
Connected Supplier Management Built for MedTech
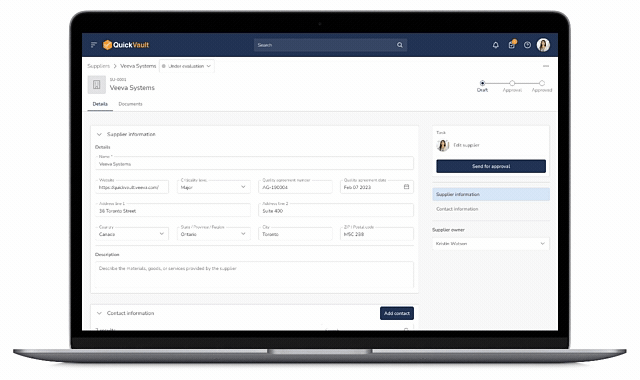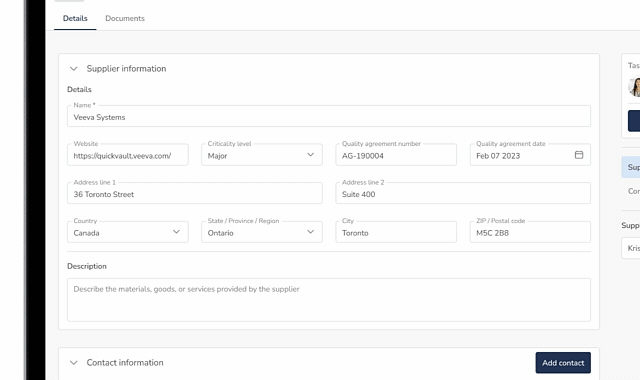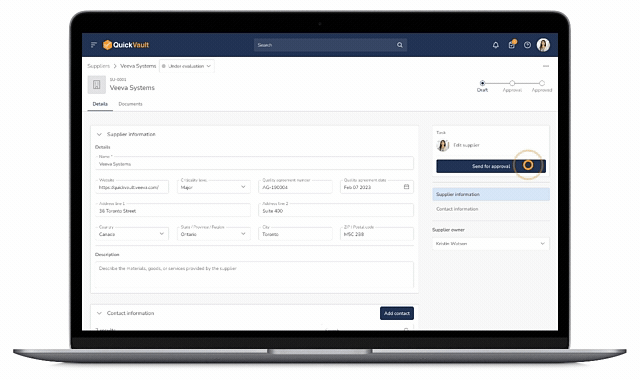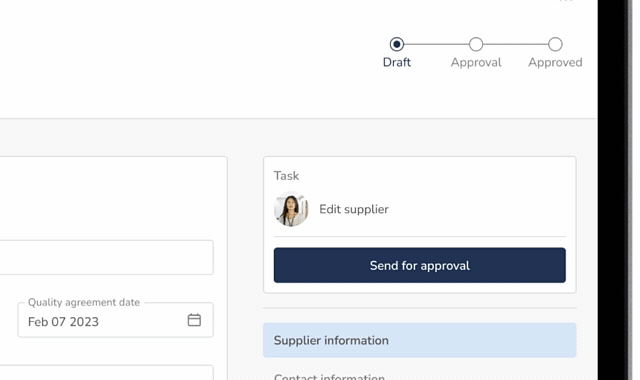
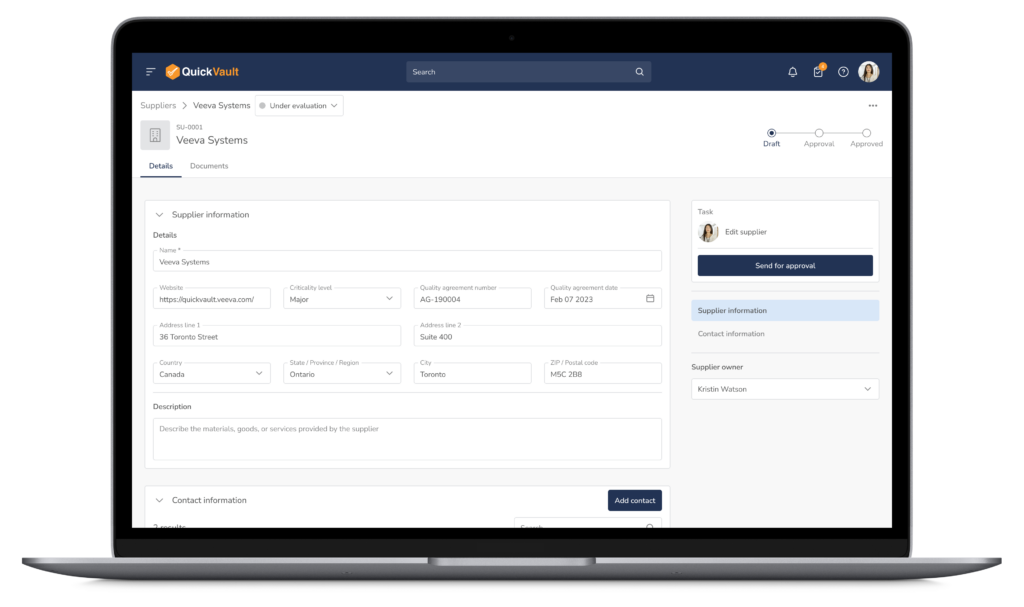
QuickVault brings supplier management, purchasing controls, and traceability into one connected system. Manage your Approved Supplier List (ASL), evaluations, and approvals while maintaining full visibility across documents, products, equipment, and quality events, all aligned with 21 CFR 820.50.
Why Supplier Management is Crucial
for Regulatory Clearance
Regulators expect MedTech companies to demonstrate control over anything that can impact product quality, performance, or safety, and suppliers are a critical part of that risk.
A structured supplier management system provides the visibility and traceability needed to prove purchasing controls, maintain supply chain oversight, and protect the integrity of your device throughout its lifecycle.
The Challenges of Medical Device
Supplier Management
Many MedTech teams still rely on home‑grown systems to manage suppliers. These often include spreadsheets for the Approved Supplier List, standalone supplier evaluation forms, and manual review cycles, creating risk and inefficiency.
Outdated & Unreliable Spreadsheets
Spreadsheets used to manage suppliers and ASLs quickly become outdated and fragmented. Manual updates and version control issues make it difficult to maintain a single source of truth, increasing audit effort and compliance risk.
Manual Supplier Approvals
Manual approvals slow supplier onboarding and introduce inconsistency. Paper forms and disconnected tools make it difficult to standardize assessments, track approval status, or maintain compliance at scale.
Disconnected Systems & Processes
Managing suppliers outside the QMS creates gaps in visibility. Without connectivity between suppliers, documents, products, and equipment, teams must manually assess impact and maintain traceability.
Time-Consuming Quality Event Investigations
When nonconformities occur, tracing affected suppliers and components often requires manual searching across systems. This delays investigations, slows corrective action, and can impact product release timelines.
Lack of Supplier-related Tracking Capabilities
Without structured supplier tracking, NCRs, CAPAs, and complaints tied to suppliers are easy to miss. Limited visibility into trends makes it harder to identify risk and prevent recurring issues.
Key Supplier Management Features
Centralized Supplier Management Software Purpose-built for MedTech
QuickVault replaces spreadsheets and disconnected tools with a single source of truth for medical device supplier management.
Connected Supplier Quality Management
- Centralize all supplier data in one system
- Link suppliers to documents, products, equipment, and quality events
- Maintain a complete supplier record across your QMS
- Gain full visibility into supplier relationships and dependencies
Purchasing Controls & Change Control with Full Traceability
- See which suppliers support specific products and components
- Perform impact analysis during change control activities
- Identify suppliers requiring retraining or updated documentation
- Maintain purchasing controls through design and process changes
Audit‑Ready Medical Device Supplier Management
- Demonstrate compliance with 21 CFR 820.50 purchasing controls
- Show complete supplier histories and approval records
- Trace suppliers to products and quality events
- Reduce audit preparation time and manual evidence gathering
Proactive Supplier Quality & Risk Reduction
- Link NCRs, CAPAs, and complaints directly to suppliers
- Track and trend supplier-related quality issues over time
- Identify patterns and take corrective action earlier
- Strengthen supplier performance and reduce recurring issues
Frequently Asked Questions About Medical Device Supplier Management
What is supplier management in medical devices?
Supplier management in medical devices ensures that all suppliers meet specified requirements for quality, safety, and regulatory compliance throughout the product lifecycle.
How does QuickVault support 21 CFR 820.50 purchasing controls?
QuickVault centralizes supplier evaluations, approvals, and approved supplier lists (ASLs) in a structured system aligned with 21 CFR 820.50 purchasing controls.
Can QuickVault replace spreadsheets for managing suppliers?
Yes. QuickVault replaces manual spreadsheets with a centralized, real-time system for managing suppliers, approvals, and purchasing controls.
How are suppliers connected to documents and products in QuickVault?
Suppliers can be linked directly to documents, products, equipment, and technical documentation, providing full traceability across the QMS.
Can supplier-related quality events be tracked in QuickVault?
Yes. NCRs, CAPAs, and complaints can be linked to suppliers, enabling tracking, trending, and proactive supplier quality management.
Does QuickVault support supplier change control?
Yes. Supplier linkages help teams assess impact during design or process changes and maintain purchasing controls during change control activities.
Can QuickVault scale as our supplier network grows?
QuickVault is designed to scale with growing MedTech teams, supporting increased supplier complexity without added manual effort.
Why MedTech Teams Choose QuickVault
One Connected Platform
Manage suppliers, purchasing controls, and supplier quality in one system.
Built for FDA Readiness
Prove control of your device, quality system and risks regulators look for.
Faster Time to
Market
Reduce delays, respond to changes faster, accelerate time to market.