Training Management Software for MedTech
Stay Audit Ready & Operate With Well-Trained Employees
Manage employee training, competency, and audit readiness without spreadsheets or manual tracking.
Built to Meet FDA 21 CFR 820 and EU MDR/ ISO 13485 Training Requirements
Discover a built-in training management system within QuickVault’s all-in-one QMS platform for medical device companies.
MedTech teams can easily assign, track, and document training and competency, while maintaining full alignment with global regulatory requirements.
Why Training Management is a
Critical Compliance Risk for MedTech
In MedTech, training gaps are compliance gaps. Regulators expect clear, documented evidence that the right people are trained on time, and on the correct versions of documents, making training management a high-risk area during inspections and audits.
The Challenges of Training MedTech Teams
Managing training and employee competency across evolving documents, teams, and regulations is a growing challenge for MedTech companies. In MedTech, when teams aren’t properly trained, the impact goes far beyond internal inefficiencies, it becomes a serious compliance, quality, and business risk.
Keeping Training Current Across Teams
As new and revised documents are released, ensuring the right employees are trained on time can quickly become unmanageable.
Missed or delayed training creates compliance gaps that are often flagged during audits.
Identifying Who Needs Training
MedTech organizations often struggle to determine which employees are impacted by new documents or revisions.
Without clear visibility into roles, departments, and responsibilities, critical training assignments can be overlooked.
Training Inefficiency & Risk
Poorly trained staff increase quality events, such as NCRs, CAPAs, and complaints, while reducing productivity and operational efficiency.
Lengthy, paper-based training slows onboarding and delays document updates, product changes, and organizational progress.
Connecting Training Gaps to Quality Events
When training deficiencies contribute to deviations, CAPAs, or other quality events, it’s often difficult to trace the root cause.
Disconnected systems make it hard to link training records to quality issues and corrective actions.
Demonstrating Competency During Audits
Regulators increasingly expect more than proof of training completion—they require evidence of employee competency.
Manually assembling training and competency records is time-consuming and error-prone, leading to missed steps, duplicate work, and slower submission readiness.
Accelerate Your Business with Simplified Training
In MedTech, business effectiveness depends on having properly trained staff who are trained correctly and trained on time. When training is slow, manual, or fragmented, it creates delays in product changes, quality processes, and onboarding that directly impact growth.
By simplifying training management, MedTech teams ensure employees are always trained on the right procedures at the right time. The result is fewer quality events, faster change implementation, quicker onboarding, and an organization that can scale with confidence, without sacrificing compliance.
Key Training Management Features
Training That Keeps Your MedTech Team Ahead
From document-driven training to fast, efficient, and audit-ready training records, QuickVault brings speed, simplicity, transparency, and connectivity to training management.
Document-Driven Training
- Automatically route new and revised documents for training
- Ensure training is completed before documents become effective
- Maintain traceability between documents and training records
Training Groups & Curricula
- Create departmental training groups (Quality, R&D, Manufacturing, etc.)
- Build training curricula (sets of required documents)
- Assign training to individuals or groups with ease
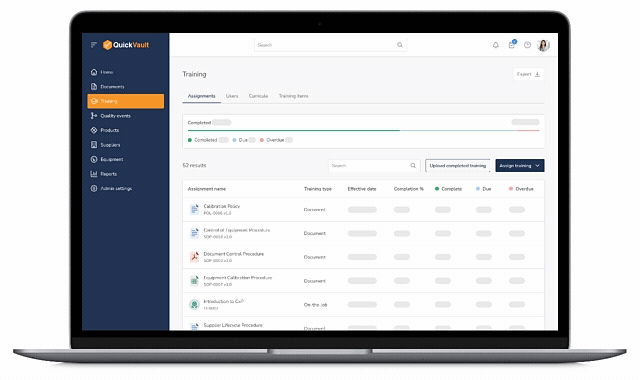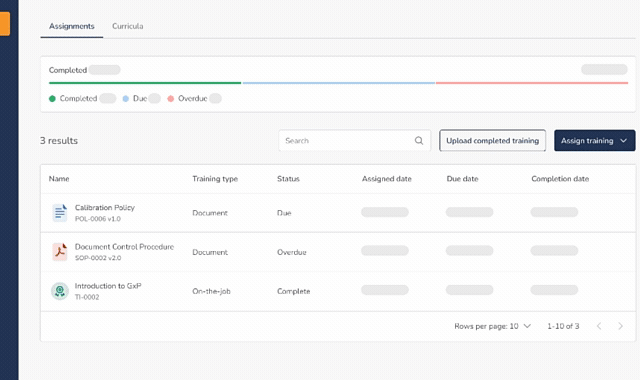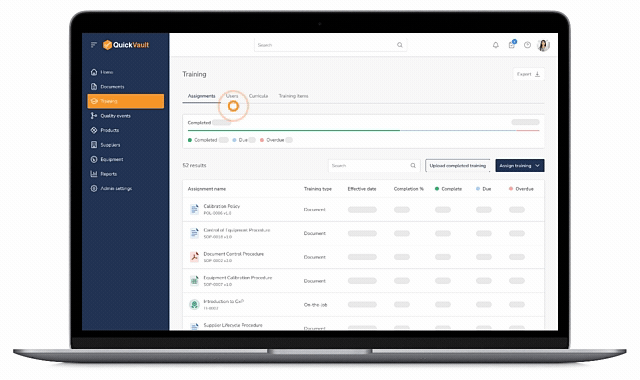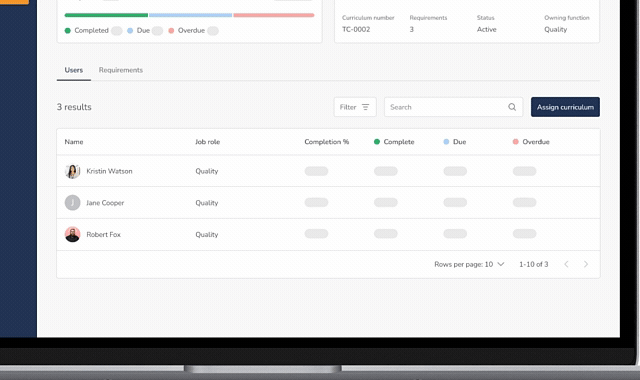
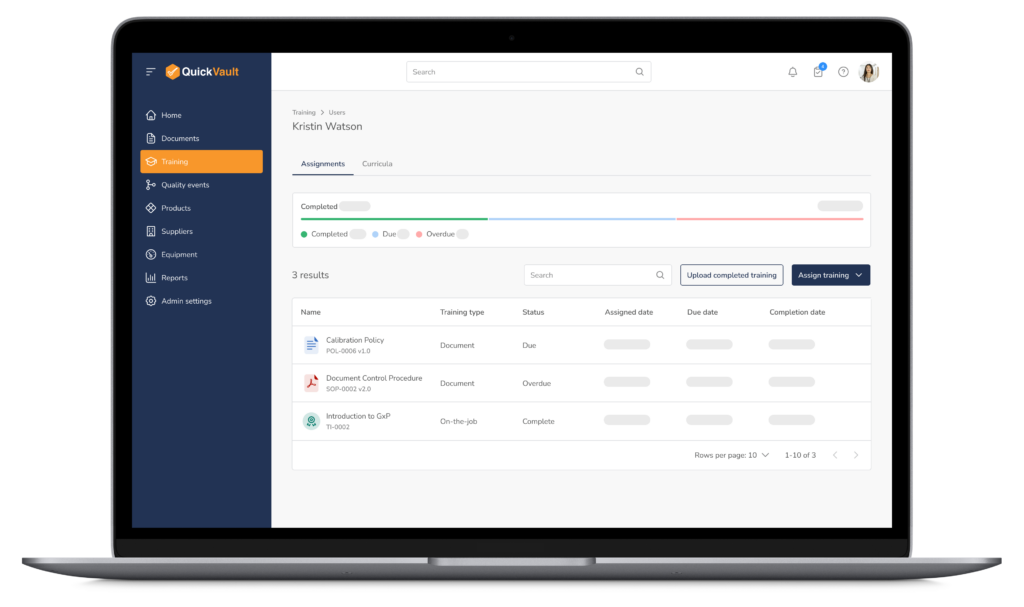
Training Dashboard & Visibility
- Central dashboard showing: Assigned training, completed training, and overdue training
- Quickly assess training status across the organization
Notifications & Accountability
- Automatic notifications when training is assigned
and when training is due - Reduce missed training and manual follow-ups
Streamlined Training Workflows
- Reduction in issues / quality events with better trained staff
- Increased efficiency in an organization with well-trained staff
- Shorter time to implement changes through more efficient training
- Support company growth with easier and more efficient onboarding of new employees
Audit-Ready Training Records
- Maintain complete, traceable training histories
- Build employee competency files that stand up to FDA, ISO 13485, and EU MDR audits
Frequently Asked Questions About Training Management
What is a training management system?
A training management system helps MedTech teams organize, assign, and track training activities across departments, ensuring compliance,streamlined learning, and teams that operate highly trained and efficient.
How do I create training groups within QuickVault?
You can create departmental or role-based training groups (e.g., Quality Team, R&D Team) and assign curricula to individuals or groups for easy management.
Can I track training completion and progress?
Yes. QuickVault’s intuitive training dashboard allows managers to monitor who has completed assigned courses, track overdue training, and generate compliance reports.
How do I ensure teams are trained on new or revised documents?
Route documents for pre-release training to make sure all teams review updates before they become effective, maintaining compliance and accuracy.
Can I assign training automatically?
Yes. Assign training to individuals or groups based on roles, departments, or document updates, and automate reminders for timely completion. Staff will get notifications when training is assigned, and when it’s due.
Is training completion tied to regulatory compliance?
Absolutely. Documented training and completion records help demonstrate compliance with FDA and other regulatory requirements for MedTech companies.
Why MedTech Teams Choose QuickVault
One Connected Platform
Clear, easy-to-use platform for viewing, assigning and completing training.
Built for FDA Readiness
Clear documentation of training proves FDA readiness for MedTech teams.
Shorter Time to
Market
Reduce manual handoffs and accelerate your approval process.