Medical Device Design Control & Risk Management Software
Manage Design and Risk with Confidence
Build the design matrix, trace to design outputs, verification, validation, and defend every design decision from concept through submission.
Take Control of Design and Risk from Day One
QuickVault unifies design controls and risk management in one connected platform, helping MedTech teams move faster while staying fully traceable and submission-ready.
Manage products, user needs, design inputs, risks, and DV&V in a single workspace. Built-in design review and risk review workflows, and automated linkages replace spreadsheets, enabling clear traceability across documents, suppliers, equipment, quality events, and submissions.
The Challenges of Managing Design Controls & Risk Management in MedTech
Design control and risk management are among the most complex and audit-scrutinized processes in medical device development. In medtech submissions, gaps from design and development are the most common issues with submission rejection and delays. When auditors review design and development activities, missing linkages or unclear traceability can quickly turn into findings.
Design Control Complexity
Design control projects are large, complex and multi-year efforts that involve many moving parts, stakeholders, and dependencies.
Without prior experience, it’s easy to lose structure, miss critical steps and significantly delay device commercialization.
Limited Knowledge & Experience
There is no one-size-fits-all playbook for medical device design controls.
Teams without deep experience and limited support struggle to know how to follow design control regulations, including risk management.
Disconnected Spreadsheets & Documents
Design controls are often managed across spreadsheets and documents that are not connected, and that constantly change.
Keeping approvals, revisions, and linkages aligned becomes time-consuming and error-prone.
Weak Traceability for Audits & Submissions
Auditors expect clear evidence linking user needs, risks, testing, and results.
Missing or unclear traceability can delay submissions, risk product commercialization timelines, and lead to audit findings.
Core Design Control & Risk Management Capabilities
Where Design Controls and Risk
Management Come Together
QuickVault provides a purpose-built Design Control and Risk Management module designed for medical device companies navigating FDA and ISO requirements.
Product Workspace
Create and manage products directly in QuickVault, serving as the foundation for design controls, risk management, and regulatory submissions.
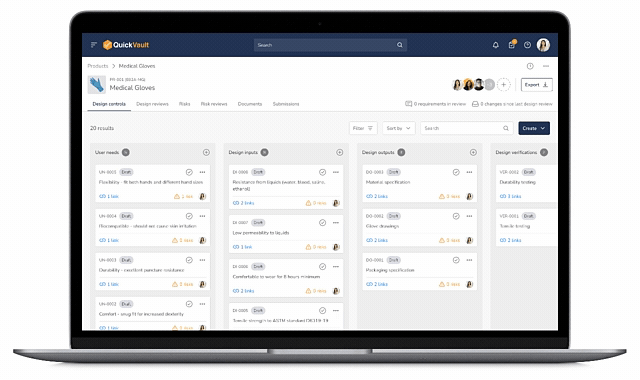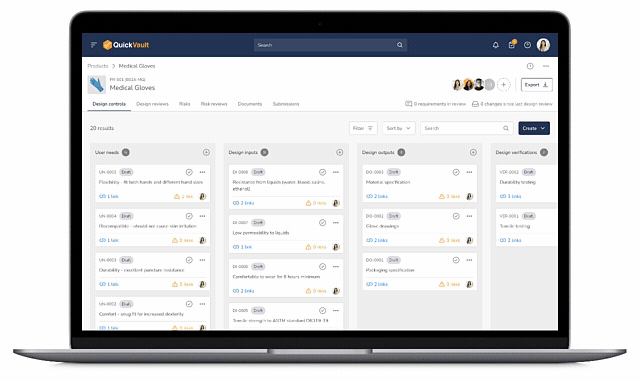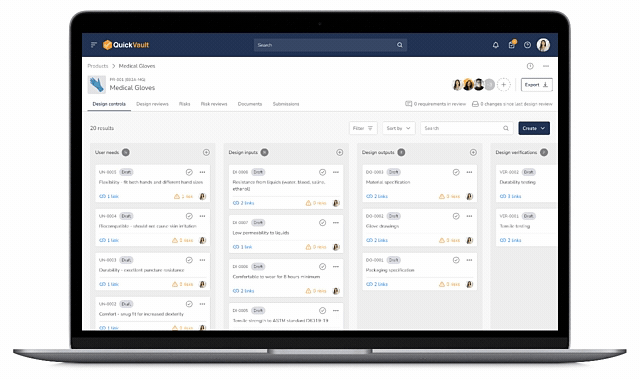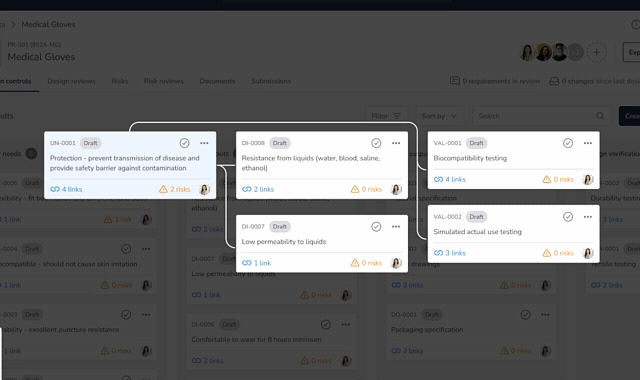
Design Control Matrix (DCM)
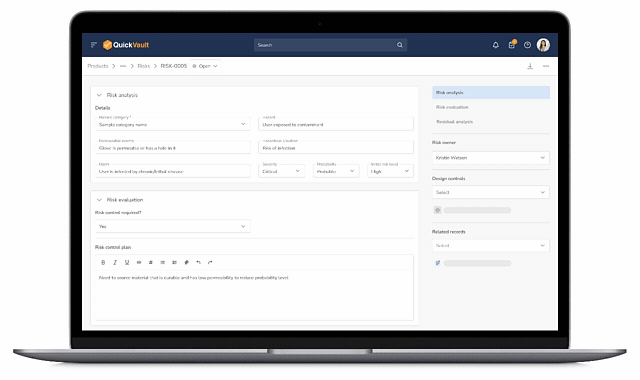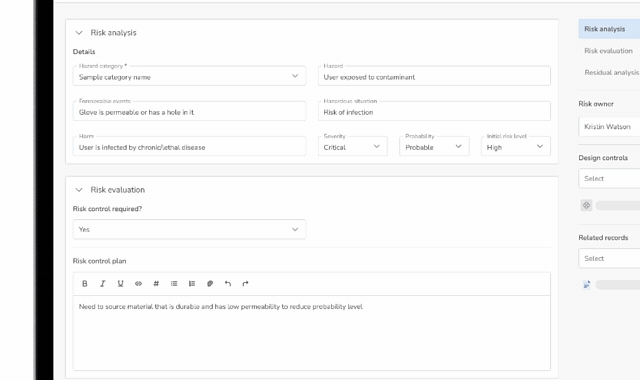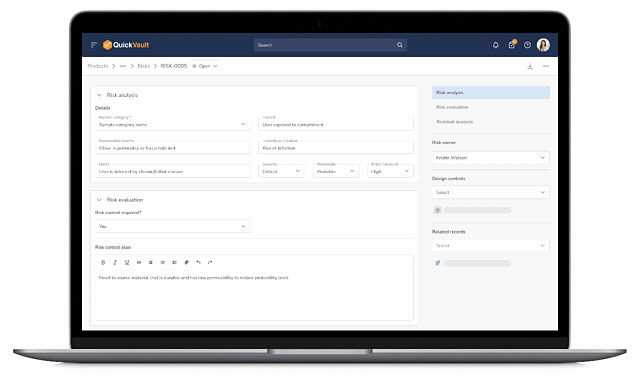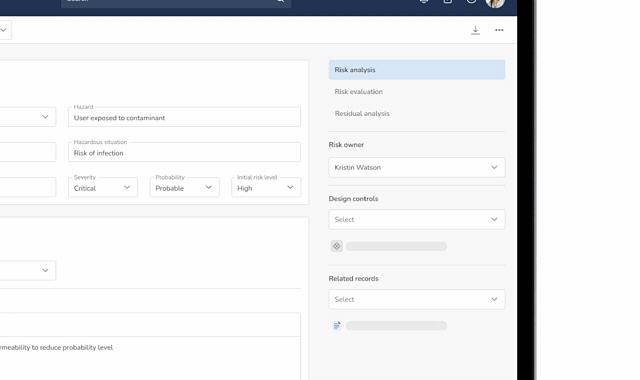
Manage user needs, design inputs, outputs, verification, and validation in a structured Design Control Matrix with full traceability.
Risk Management (ISO 14971)
Identify, assess, and mitigate risks using a dedicated risk management module that links directly into the Design Control Matrix.
Document & Object Linkages
Connect design controls to documents, suppliers, quality events, equipment, and other QuickVault objects, eliminating manual cross-referencing. Leverage 21 CFR Part 11 compliant electronic signatures for fast signoff of documents and Design Control items.
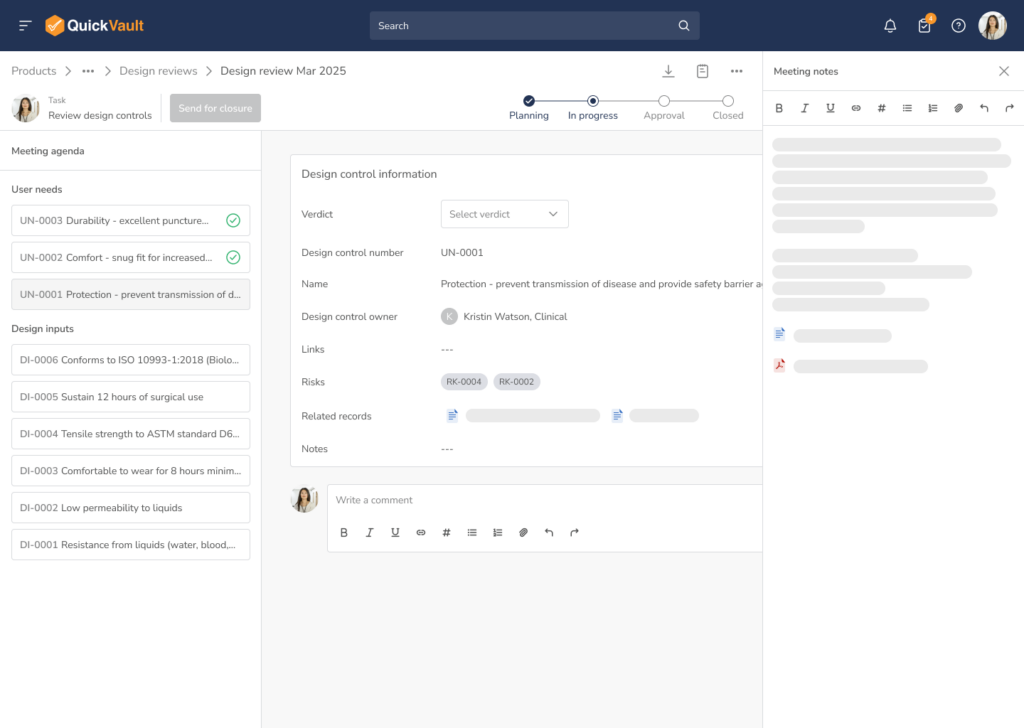
Design & Risk Reviews
Run formal Design Review and Risk Review meetings with documented approvals and full audit trails.
Interactive Traceability Map
Visually click-through to trace relationships between user needs, risks, design inputs, verification, validation, and documentation.
Connection to Manufacturing Equipment
For physical medical devices, products have to be built to allow for design verification and design validation testing. Per medtech regulations, any equipment used for such builds have to be documented and under control.
QuickVault’s Equipment module connects to the Products module, allowing for transparency and linkages between design inputs and risks, to equipment used for product builds. This allows for full traceability between products used for DV&V testing, and the final design intended for commercial distribution.

Key Workflows within QuickVault
Built for Regulatory Submissions and Audits
Most design control outputs are reused across regulatory submissions such as 510(k), PMA, De Novo, and CE Mark applications. QuickVault enables teams to connect design controls, risk management records and documents in one centralized platform. With everything linked and searchable, submission creation and audits become faster, smoother, and far less stressful.
- Populate structured design files (Design & Development File and the Medical Device File ) with a single click
- Maintain a complete history of the device throughout development
- Tie design controls directly into the Regulatory module to prepare submissions in parallel
- Sign off electronically for all documents while staying 21 CFR Part 11 compliant
- Provide auditors with clear, traceable evidence of design decisions, testing, and risk mitigation
Frequently Asked Questions About Medical Device
Design Control and Risk Management
What is medical device design control software?
Medical device design control software helps manufacturers manage design and development activities required by MedTech regulations such as FDA 21 CFR 820.30 and ISO 13485, including user needs, design inputs, outputs, verification, validation, and design reviews. It ensures traceability and documentation throughout the product lifecycle.
How does QuickVault support FDA design controls?
QuickVault provides a structured Design Control Matrix (DCM) that links user needs, design inputs, verification, validation, and supporting documentation. Design reviews, risk reviews, approvals, and audit trails are managed directly within the platform to support MedTech design control requirements.
What is ISO 14971 risk management for medical devices?
ISO 14971 is the international standard for risk management in medical devices. It requires manufacturers to identify hazards, assess and control risks, and monitor the effectiveness of risk controls throughout the device lifecycle.
How does QuickVault support ISO 14971 risk management?
QuickVault includes a dedicated risk management module that supports ISO 14971 workflows. Risks can be identified, assessed, mitigated, and directly linked to design controls, verification, validation, and supporting documents. A separate Risk Review workflow is available for the risk team to review risks, both during design and development, and for future product changes of customer complaint investigations.
Can design controls and risk management be linked together?
Yes. QuickVault connects design controls and risk management within a single system, allowing risks to be traced directly to user needs, design inputs, testing activities, and mitigations. This linkage supports transparency, which is a great benefit for planning design verification and design validation activities, making sure that product testing is approached with both device performance and safety in mind.
What is the Design & Development File in medical devices?
The Design & Development File is a collection of records that demonstrates a medical device was developed in accordance with approved design plans and regulatory requirements. It is a critical component of the design control regulations within the medtech industry.
How does QuickVault help create and maintain the Design & Development File?
QuickVault automatically links product documentation, design controls, risk management activities, and reviews to build a complete Design & Development File as an output from product development. A structured Design & Development file with built-in hyperlinks can be generated and exported with a single click.
Does QuickVault support design reviews and risk reviews?
Yes. QuickVault includes formal Design Review and Risk Review workflows with assigned reviewers, approvers, and a complete audit trail. This ensures design decisions are documented and compliant. As part of a design review, other items not subject to regulatory overview, such as timelines, budgets etc. can be reviewed and managed as well, providing full project review and design review capabilities.
Can design control data be reused for regulatory submissions?
Yes. Design control outputs in QuickVault can be tied directly into the Regulatory module to populate submissions such as 510(k), PMA, De Novo, and CE Mark applications in parallel. Building a submission as you go through the design and development effort minimizes the need to create a submission from scratch after the design project is concluded, ultimately leading to shorter time to submission, and earlier commercialization.
Who should use design control and risk management software?
Design control and risk management software is used by medical device quality, R&D, regulatory, and product development teams to manage design activities, mitigate risk, and maintain compliance throughout the product lifecycle.
Why MedTech Teams Choose QuickVault
Efficiency & Speed
Reduce time to market through automation and reuse.
Transparency
See how design controls and risks connect at every level.
Connectivity
Link design data to quality events, suppliers, and submissions.