Medical Device Document Control & Change Control Software
Control Documents and Changes in One Unified eQMS
Centralize document control and change management in one connected system. Ensure compliance, maintain full traceability, and eliminate manual, siloed processes.
Quality Built Into Every Document and Change
QuickVault helps MedTech teams maintain efficiency and compliance at every stage by unifying document control and change control in a single eQMS.
Built specifically for MedTech, teams have access to purpose-built workflows, electronic signatures, task assignments, and full audit trails so they can maintain speed and quality from development through commercial growth.
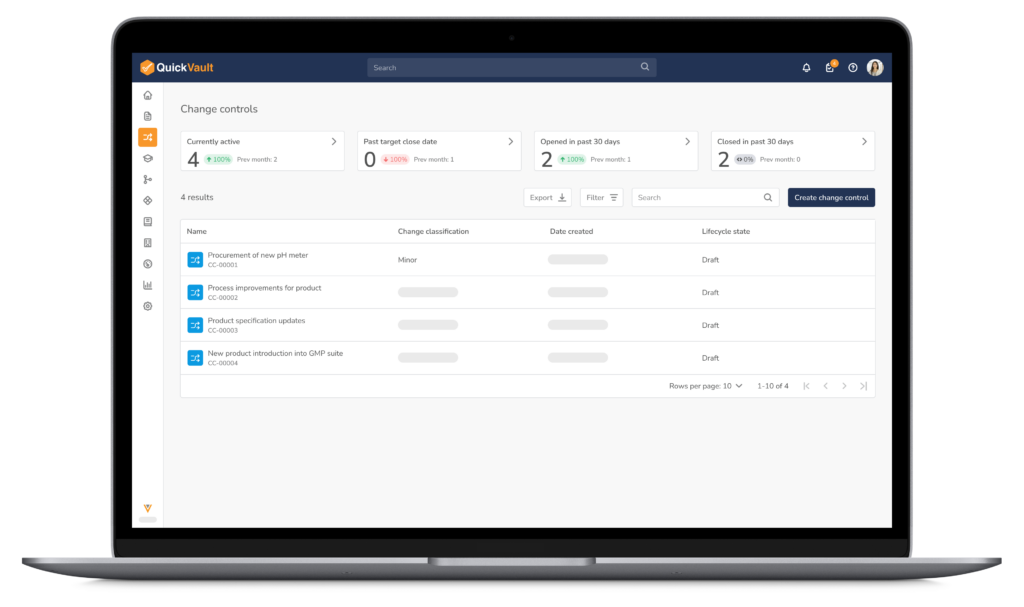
The Challenges of Document & Change Control in MedTech
Document and change control are the foundations of a medical device QMS, and among the most difficult processes to manage at scale. Without structured software to control documents and changes, teams face audit risk, operational delays, and costly compliance gaps.
Document Volume & Version Control
Medical device quality systems often contain thousands of controlled documents that evolve constantly. Without structured version control, teams struggle to track revisions and ensure only the latest approved documents are in use.
Complex & Large Changes
Large changes, such as SOP updates or ECOs, often impact multiple documents, products, and suppliers. Without automation and clear traceability, teams risk missed updates, compliance gaps, and delayed implementation.
Slow Reviews & Disconnected Teams
Cross-functional reviews often span multiple teams, locations, and time zones. Without structured workflows, approvals delay, accountability becomes unclear, and product timelines slip.
Signature & Compliance Burden
Manual signatures and third-party eSignature tools add cost and complexity. Paper-based or scanned approvals make it harder to consistently meet 21 CFR Part 11 requirements.
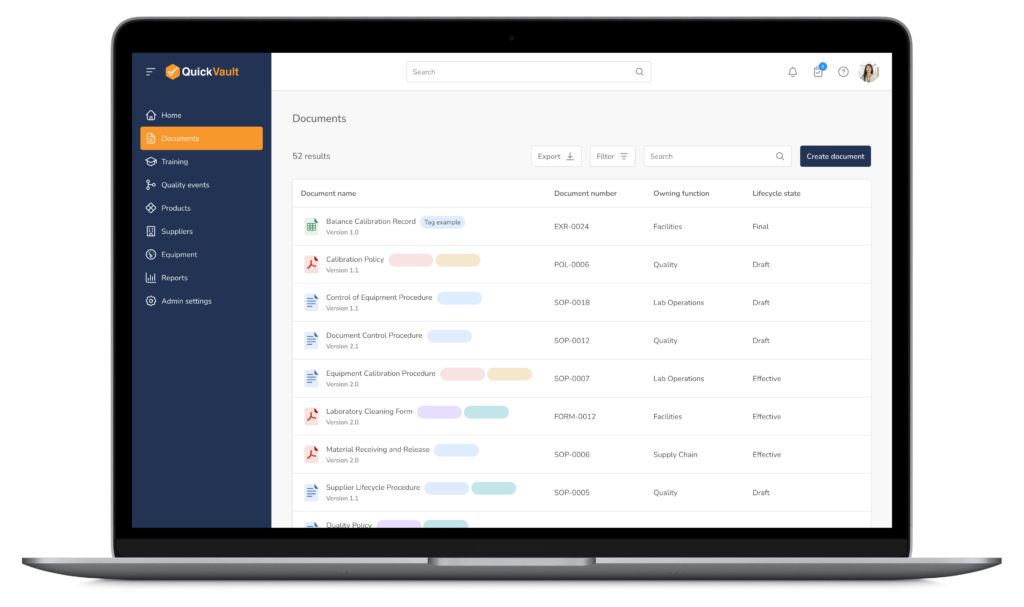
Core Document Control Capabilities
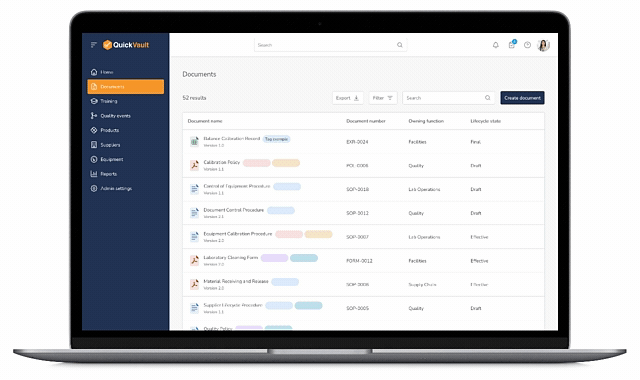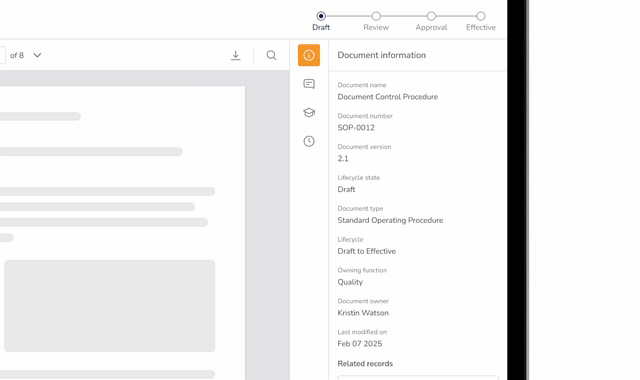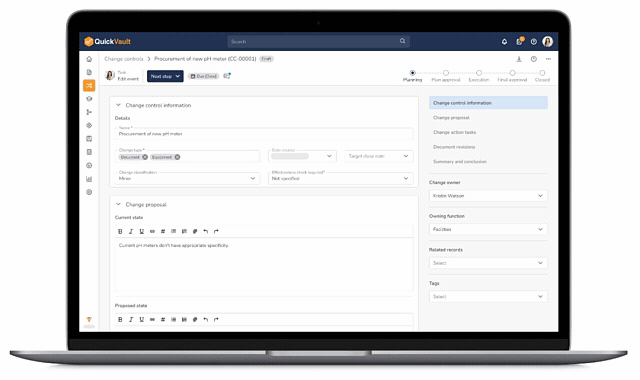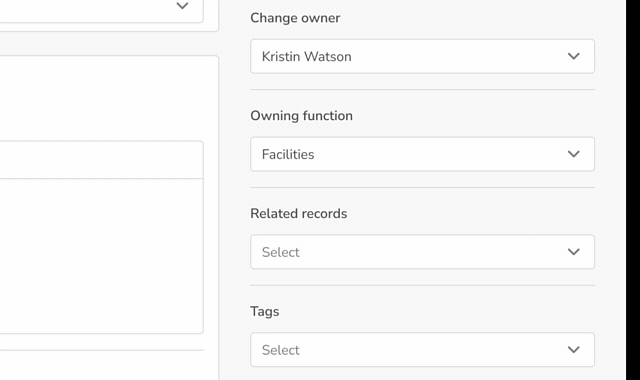
One Source of Truth for Controlled Documents
QuickVault provides purpose-built document control and change control functionality designed specifically for medical device companies.
Document Creation & Revision Control
Quickly create, revise, and manage controlled documents with clear versioning and lifecycle states.
Review & Approval Workflows
Route documents through structured review and approval workflows tailored to document type and lifecycle.
21 CFR Part 11 Electronic Signatures
Sign documents electronically using built-in, compliant eSignatures, with no paper, scanning, or third-party tools required.
Audit Trails & Watermarked PDFs
Automatically generate approved PDFs with approval names and dates, backed by a complete audit trail.
Task Visibility & Notifications
Collaborators see assigned document tasks directly on their dashboard, with automatic email notifications to keep work moving.
Always Stay Audit-Ready
QuickVault keeps documents and changes organized, searchable, and audit-ready. Built-in traceability and tagging make it easy to retrieve requested documentation in seconds.
Core Change Control Capabilities
Control Every Change with Confidence
With QuickVault, engineering changes stay connected, traceable, and compliant from start to finish.
Structured Engineering Change Orders (ECOs)
Manage complex technical changes with formal ECO workflows that guide teams from impact assessment to approval and implementation.
Change Impact Assessment
Evaluate how changes affect documents, products, parts, materials, suppliers, and processes before execution to reduce downstream risk.
Cross-Functional Task Management
Assign clear ownership and track responsibilities across Quality, R&D, Manufacturing, and Regulatory teams throughout the change project.
Connected Document & Product Linkages
Automatically identify impacted documents, specifications, drawings, and work instructions to ensure nothing is overlooked.
Approval Workflows & Audit Trails
Route changes through structured approvals with full traceability and audit-ready documentation of every decision.
Automatic Updates to Product Files
Automatically keep the Design & Development File and Medical Device File current as changes are approved and implemented.
Changes Initiated from Quality Events
When technical changes originate from Nonconformances, CAPAs, or Complaints, they can be directly linked to the originating quality event to maintain full traceability.
Frequently Asked Questions About Medical Device
Document Control and Change Control
What is document control software for medical devices?
Document control software helps medical device companies create, revise, approve, and manage controlled documents in a compliant, centralized system. It ensures proper versioning, traceability, and audit readiness across all QMS documents. It also enables faster document creation and revision while making it easy to quickly find the documents you need.
How does QuickVault support document control?
QuickVault provides structured workflows for document creation, revision, review, and 21 CFR Part 11–compliant electronic signatures. Built in alignment with FDA 21 CFR 820.40, EU MDR, and ISO 13485, the system ensures controlled approvals, full audit trails, and access to only the latest approved versions across your organization.
What is change control in medical devices?
Change control is the structured process of evaluating, approving, and implementing updates to documents, products, processes, equipment, or suppliers. For technical changes such as Engineering Change Orders (ECOs), automation ensures all impacted areas are assessed and updated to maintain compliance and avoid delays.
How does QuickVault manage technical changes?
QuickVault manages technical changes through structured Engineering Change Order (ECO) workflows that include impact assessment, cross-functional task management, and connectivity to Risk Management and Design Controls. This ensures all affected documents and records remain traceable, updated, and audit-ready.
Can documents and changes be linked together in QuickVault?
Yes. QuickVault connects documents, products, specifications, suppliers, and quality events so changes are automatically tracked across all affected items. This reduces the risk of missed approvals and maintains clear, audit-ready traceability.
How does QuickVault help with audits?
QuickVault keeps documents and changes fully traceable and inspection-ready. Advanced search, tagging, and organization make it easy to quickly locate requested records, while audit trails, electronic signatures, and automatic watermarks allow auditors to verify approvals and revisions with confidence.
Who should use document and change control software?
Document and change control software is used by R&D, Quality, Regulatory, and Manufacturing teams at MedTech companies to efficiently manage documents, technical changes, and compliance requirements. It supports cross-functional alignment, audit readiness, and streamlined implementation of changes.
Can QuickVault handle both minor document revisions and large technical changes?
Yes. QuickVault manages everything from simple document updates to complex, multi-document Engineering Change Orders (ECOs). Structured workflows ensure changes are controlled, traceable, and implemented efficiently while maintaining compliance at every stage.
Why MedTech Teams Choose QuickVault
Simplicity
Intuitive document and change workflows.
Collaboration
Seamless cross-functional review and approval.
Connectivity
Link documents to products, suppliers, equipment, and quality events.