Medical Device Quality Event Management Software
Track, Investigate, and Resolve Quality Issues Quickly
Simplify CAPA, complaint, and nonconformance management with connected, audit-ready workflows built for MedTech.
A Smarter Way to Manage Quality Events
QuickVault’s Quality Events module helps medical device companies manage Non-conformances, CAPAs, and customer complaints with structure, transparency, efficiency, and full traceability.
Say goodbye to spreadsheets, silos, delayed issue resolutions, and audit stress.
The Challenges of Managing Quality Events in Medical Devices
Managing quality events in the medical device industry is complex and critical. Thus, manual processes only increase risk in several ways. Without the right quality event management software, these gaps can result in audit findings, delayed field actions, delayed product release, recalls, or increased risk of patient and user harm.
Manual Quality Event Initiation & Logging
Initiating, logging and managing quality events manually slows evaluation and increases the risk of incomplete or inconsistent data.
Delays at this stage can stall investigations, corrective actions, and product release.
Disconnected Investigation Data
Identifying affected products, parts, or equipment is difficult when information lives in separate systems.
Missing linkages make root cause analysis more time-consuming and error-prone.
Unclear Ownership & Accountability
Without defined workflows, it’s often unclear who owns each step of an investigation or corrective action.
This leads to missed handoffs and delayed quality event closure.
Audit Gaps & Inconsistent Documentation
Incomplete or inconsistent quality event records are often uncovered during audits.
These gaps increase the risk of regulatory findings and inspection stress.
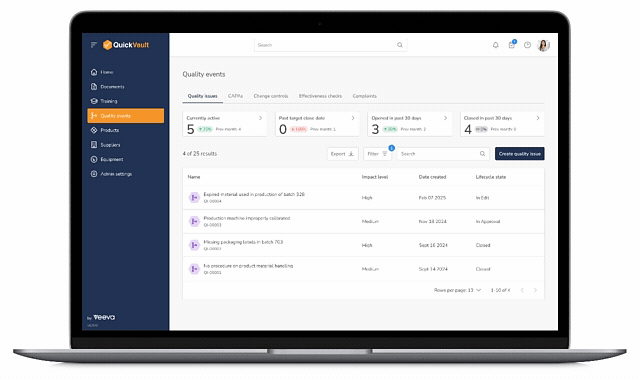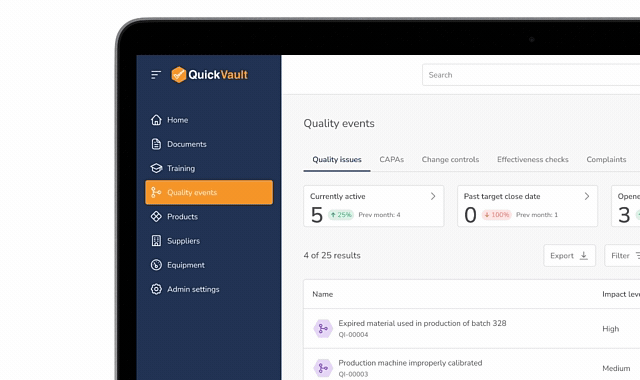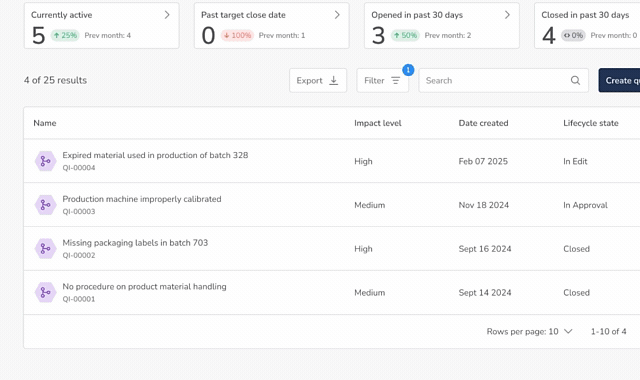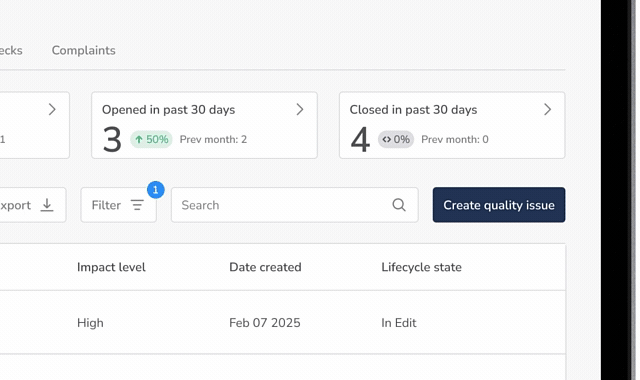
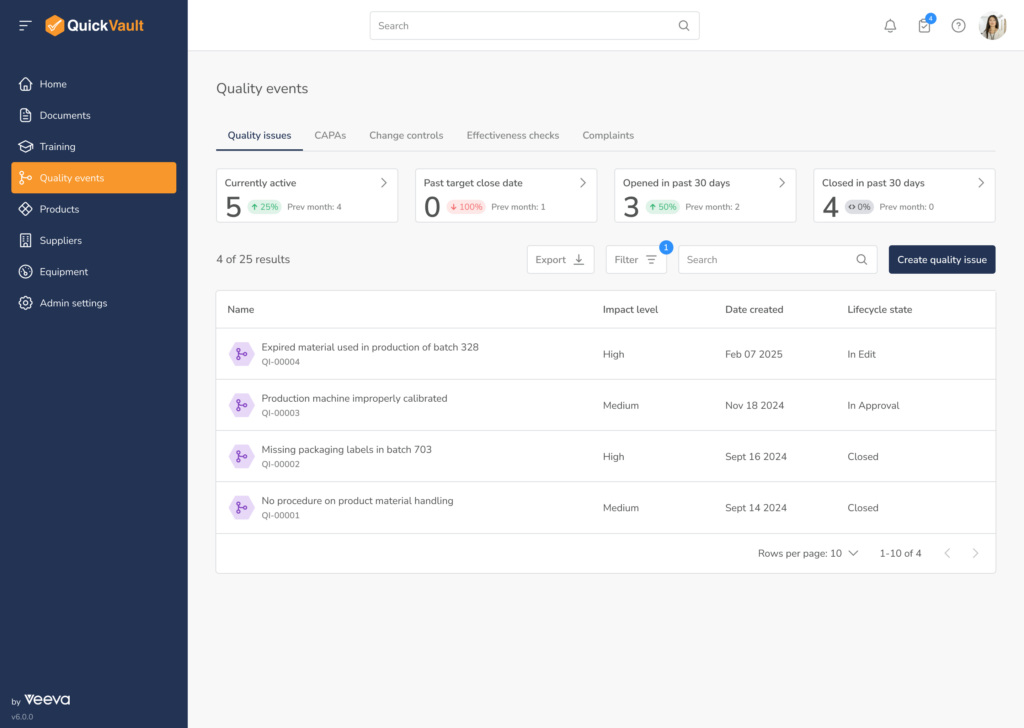
Core Quality Event Workflows
One Connected System for All Quality Events
QuickVault’s Quality Events module provides automated, purpose-built workflows to manage the full lifecycle of medical device quality issues.
Quality Issues & Nonconformances
Log, investigate, and manage deviations and nonconformances with structured workflows that ensure speed, completeness and consistency.
CAPA Management
Dedicated CAPA management software for medical devices designed to support corrective and preventive actions, root cause analysis, effectiveness checks, and closure.
Customer Complaint Management
Track and assess device failures in the field with complaint management software built for regulatory compliance, including evaluation of adverse event reporting requirements.
Effectiveness Checks
Verify that corrective actions are effective over time and fully documented, reducing repeat nonconformances and audit risk.
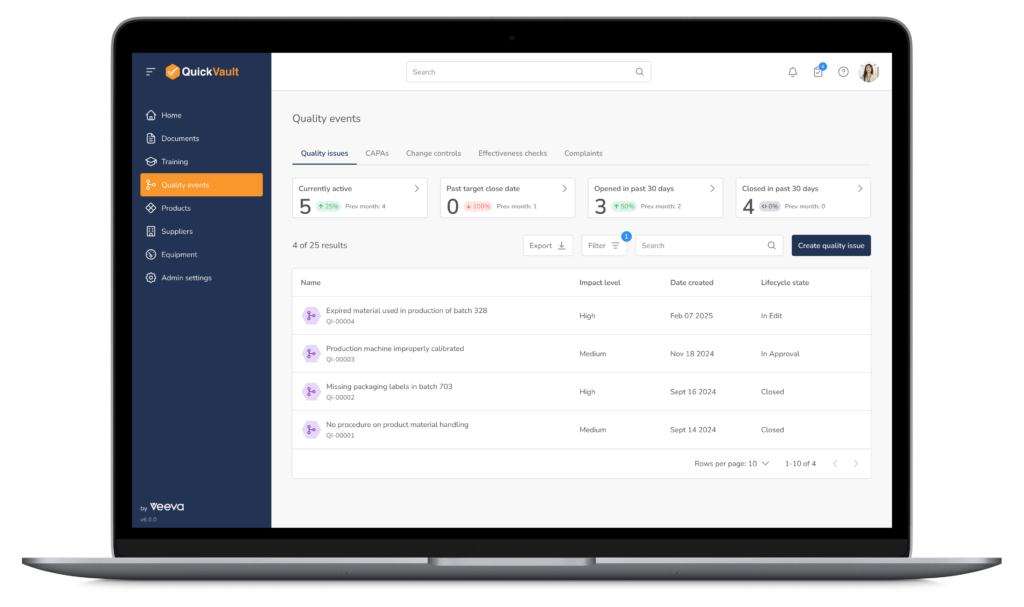
Key Quality Event Management Capabilities
Quality Event Management, the QuickVault Way
QuickVault enables teams to manage quality events with clarity, control, and connectivity
to support faster investigations, stronger root cause analysis, and confident decision-making.
- Automated workflows that ensure critical information is captured consistently
- Task assignment and collaboration, clearly defining responsibilities across CAPAs and complaints
- End-to-end connectivity between quality events, products, suppliers, equipment, batch records, and documentation providing transparency to all affected areas
- Tracking and trending of quality events to identify recurring issues and trigger CAPAs to improve quality and reduce risk of recurrence
- Complete documentation to support audits, inspections, and regulatory submissions
Frequently Asked Questions About Medical Device
Quality Event Management
What is medical device quality event management software?
Medical device quality event management software helps manufacturers track, investigate, and resolve quality issues such as nonconformances, deviations, CAPAs, and customer complaints in a structured, compliant system. It supports documentation, traceability, efficiency, and audit readiness across the full quality event lifecycle.
How does QuickVault support CAPA management in medical devices?
QuickVault provides a dedicated CAPA management workflow designed specifically for the medical device industry.Teams can document root cause analysis, assign corrective and preventive actions, perform effectiveness checks, and track CAPA closure all with full traceability and audit-ready documentation.
Can QuickVault manage medical device customer complaints?
Yes. QuickVault includes complaint management software built for medical devices, enabling teams to log and investigate customer complaints, assess device failures in the field, and evaluate whether an adverse event requires reporting to regulatory authorities. Customer complaints can be connected to subsequent CAPAs, to manage any efforts to implement changes that will prevent future field failures.
How does QuickVault help manage nonconformances and deviations?
QuickVault enables teams to manage nonconformances and medical device deviations using automated workflows that ensure consistency and completeness. These quality events can be linked to affected products, suppliers, equipment, and documentation to accelerate investigations and any corrective actions.
Does QuickVault support root cause analysis for medical devices?
Yes. QuickVault supports structured root cause analysis by connecting quality events to relevant data such as batch records, work instructions, specifications, and equipment. This connectivity helps teams identify systemic issues and implement effective corrective actions.
How is Risk Management in QuickVault tied to Quality Events?
Within QuickVault’s Risk Management module, risks associated with the design, manufacture, distribution, use and disposal of medical devices can be managed. When a quality event occurs, a Risk Review can be launched within QuickVault, to assess the current risk management file, and have that documented as part of the quality event investigation.
How does QuickVault improve tracking and trending of quality events?
QuickVault captures quality event data in a centralized system, making it easy to track open and closed events, identify recurring issues, and trend nonconformances over time. These insights help determine when a CAPA should be initiated and support continuous improvement.
Is QuickVault suitable for FDA & Notified Body audits and inspections?
QuickVault is designed to support audits by medical device regulatory agencies by ensuring quality events are fully documented, traceable, and consistent. This reduces the risk of audit findings related to incomplete records, missing linkages, or unclear corrective actions.
Can quality events be linked to products, suppliers, and equipment?
Yes. QuickVault connects quality events to products, suppliers, equipment, batch records, and documentation. This transparency helps teams quickly identify affected areas and assess risk during investigations, recalls, or field actions.
Who should use medical device quality event management software?
Medical device manufacturers, quality teams, regulatory professionals, and operations teams gain significant efficiency and speed from a quality event management software. A MedTech specific purpose-built software solution to manage quality issues, CAPAs, complaints, and deviations promotes compliance and keeps companies audit-ready.
Why MedTech Teams Choose QuickVault
Simplicity
Intuitive workflows without unnecessary complexity.
Connectivity
Link quality events to the data that matters.
Transparency
Full traceability across products, suppliers, and processes.