Elevate Your MedTech Startup
Bringing a medical device to market is an uphill battle—complex regulations, compliance hurdles, and approval delays can stall even the most promising innovations. QuickStart eliminates these roadblocks with a fully validated, intuitive Device-to-Market Platform designed specifically for MedTech startups.
With QuickStart, you can move fast, meet FDA and ISO requirements with confidence, and position your device for commercial success—all without getting lost in compliance complexity.
Learn More About QuickStart
What is QuickStart?
A compliance shortcut without the shortcuts. QuickStart is a ready-to-use regulatory and quality solution that gives MedTech startups the structure you need to launch, scale, and sustain success in the market.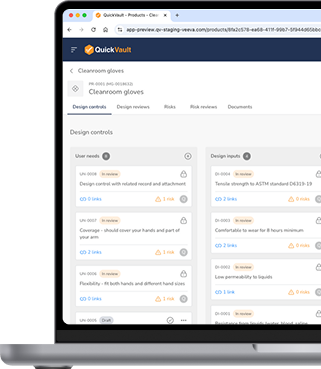
What's Included in QuickStart?
- Fully Validated eQMS: Pre-configured with FDA, ISO, and GxP best practices, eliminating implementation and setup time.
- Comprehensive Documentation Package: Pre-built, customizable SOPs, templates, and training materials included for faster approvals
- Regulatory & Quality Support: 5 hours per month of dedicated QA/RA expertise to help your team navigate compliance challenges and avoid pitfalls.

Why Choose QuickStart?
QuickStart isn’t just about getting your device to market—it’s about keeping it there. While other systems stop at submission, QuickStart supports your device through every stage of its lifecycle, ensuring regulatory compliance and operational excellence long after approval.

No setup, no gaps, no wasted time. QuickStart is ready to use immediately, giving you a validated foundation from Day 1.

QuickStart keeps every required document organized, automates workflows, and ensures submission readiness—helping you avoid costly submission mistakes and approval delays.

From early-stage compliance to full-scale production, QuickStart grows with your company—so you never have to switch systems or start over as your needs evolve.
Frequently Asked Questions
-
Who is QuickStart for?
MedTech startups looking to avoid compliance pitfalls, accelerate approvals, and scale without unnecessary regulatory burdens.
-
Do I need regulatory experience to use QuickStart?
No. QuickStart provides everything you need, including an easy-to-use eQMS, pre-built documentation, and expert support, so you can stay focused on innovation.
-
How does QuickStart help with regulatory submissions?
QuickStart keeps all required documentation organized, tracks submission progress, and streamlines regulatory interactions to get you to market faster.
-
Can QuickStart scale as my startup grows?
Yes. QuickStart is designed to support your entire MedTech journey, from early design and compliance needs to full-scale production.
Launch Faster. Scale Smarter. Stay Compliant.
Your competition isn’t waiting—and neither should you. QuickStart provides MedTech companies with the compliance foundation it needs to bring devices to market and keep them there.