Medical Device Regulatory Submission Software
Supercharge
Your Medical Device Regulatory Submissions
Prepare, track, and submit your MedTech submissions easier and faster in one connected platform built for US, EU, and global markets.
Accelerate the Path from Submission to Approval
Managing regulatory submissions doesn’t have to be manual or fragmented. Whether you are pursuing a pre-submission, an IDE, a 510(k), PMA or a CE-mark, QuickVault gives MedTech teams one connected solution to prepare, track, and submit regulatory filings, ensuring compliance and accelerating approvals.
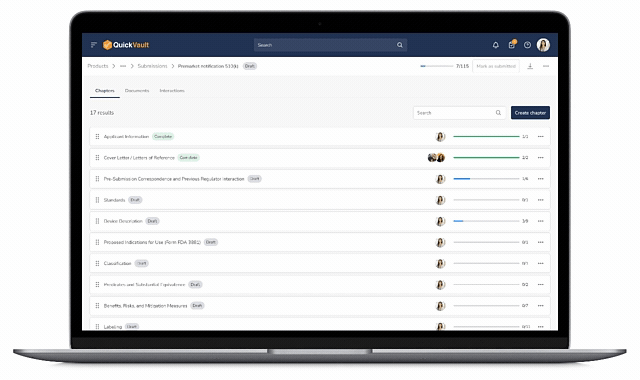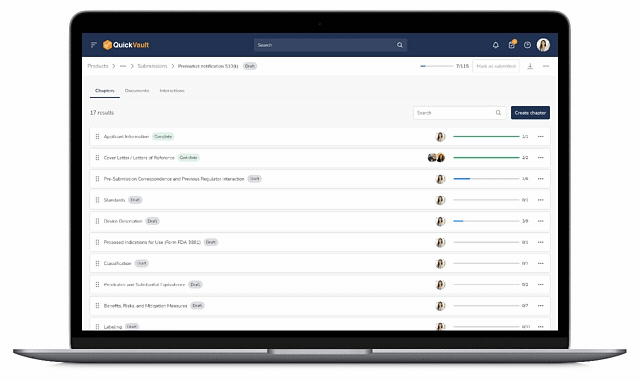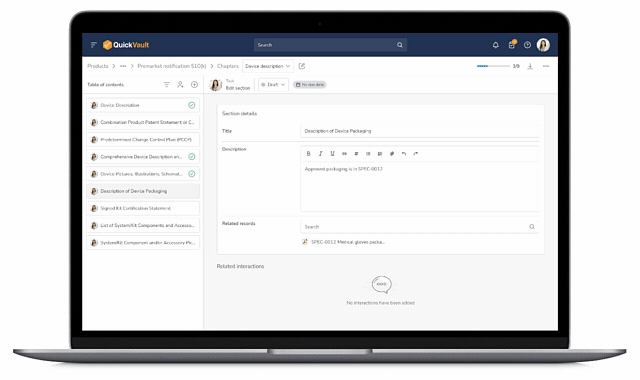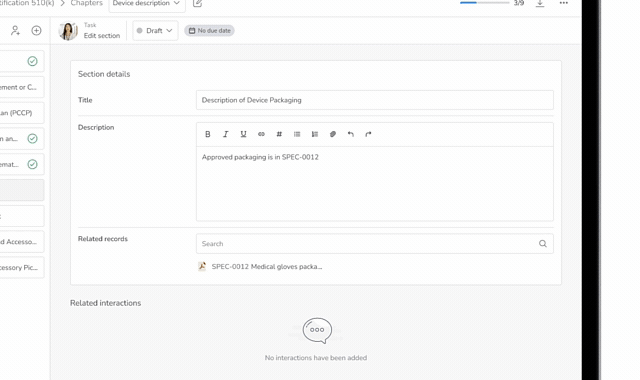
The Problem with Regulatory Submissions
for MedTech Teams
Regulatory submissions are often slowed by fragmented and disconnected systems, duplicated documents, and unclear roles across teams. After submission, there is limited visibility into questions received from regulatory authorities. Manual tracking is often done to respond to those questions, which complicates a timely response and receiving insights to document revisions. This will ultimately delay approvals for MedTech teams. Below are just some of the challenges MedTech teams face when dealing with their regulatory submissions.
Scattered Submission Content
Most MedTech teams rely on a mix of documents, spreadsheets, email, and shared drives, leaving critical submission content spread across multiple tools.
This fragmentation leads to duplicated files, outdated versions, and confusion about which documents belong in the final submission package.
Unclear Roles & Manual Project Tracking
Most MedTech teams rely on a mix of documents, spreadsheets, email, and shared drives, leaving critical submission content spread across multiple tools.
This fragmentation leads to duplicated files, outdated versions, and confusion about which documents belong in the final submission package.
Disconnected Documentation
In most MedTech regulatory submissions, product specific information such as parts of the Design & Development file, has to go into the submission. Such documents, like verification & validation reports, risk files, and other design control items, often live in separate systems.
To create the submission, teams thus end up manually copying these documents into submission folders such as Dropbox etc., creating version-control issues when updates are made elsewhere.
This disconnection makes it difficult to maintain accuracy and ensure that there are no gaps within the submission, and making sure everything aligns with regulatory expectations.
Limited Visibility with Regulatory Authorities
Following the submission, regulatory authorities often provide their feedback and questions through a single document provided via email. This can create transparency issues across the team with regards to any submission deficiencies that have to be addressed in a timely manner.
Combined with manual tracking of document revisions following the response to the questions from a regulatory authority, it’s easy to miss deadlines or lose the audit trail from submission to approval.
These gaps can delay approval or clearance and strain relationships with regulatory bodies.
A Regulatory Submission Solution
Purpose-built for MedTech
QuickVault brings every part of the regulatory submission process into one connected workspace.
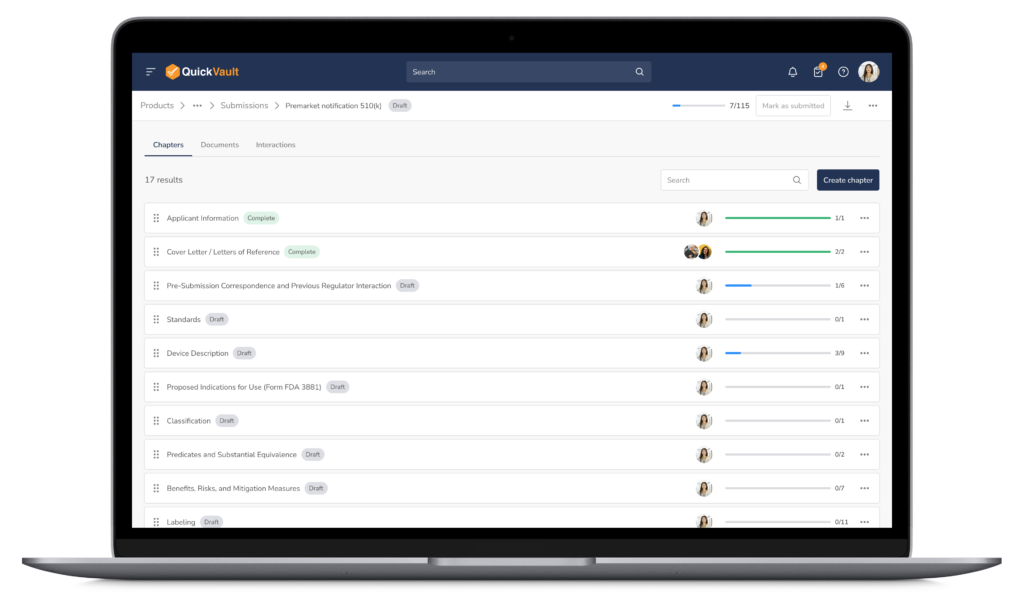
Centralized Submission Management
Teams can create, organize, and update submission content without juggling multiple tools or worrying about version control.
End-to-End Traceability
Design and development documentation is linked directly from the Products module into the submission, ensuring accuracy and eliminating manual work.
Integrated Workflow & Task Management
Clear ownership, real-time collaboration, and built-in task management keep everyone aligned.
Submission Status Tracking
With centralized tracking and visibility into the questions received from regulatory bodies, teams never miss response deadlines, streamlining the path from submission to approval.
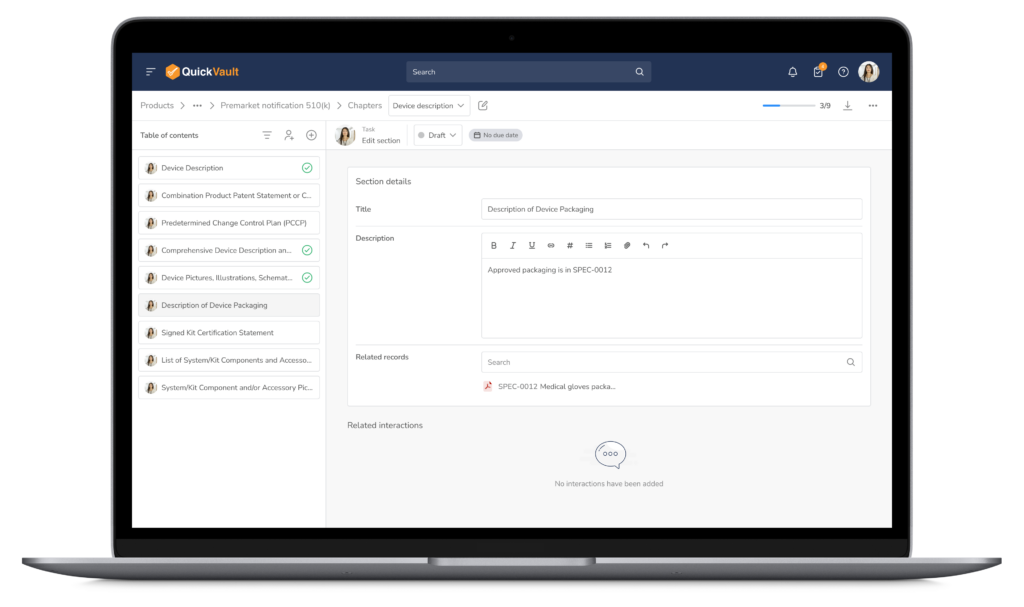
Key Capabilities within QuickVault
Everything You Need to Prepare, Submit, and Track — in One Place
- Submission templates aligned with FDA eSTAR and MDR formats (510(k), PMA, CE mark etc.)
- Possibility to create any submission from scratch outside the most common submission types
- Centralized version control and traceability
- Real-time collaboration and document review
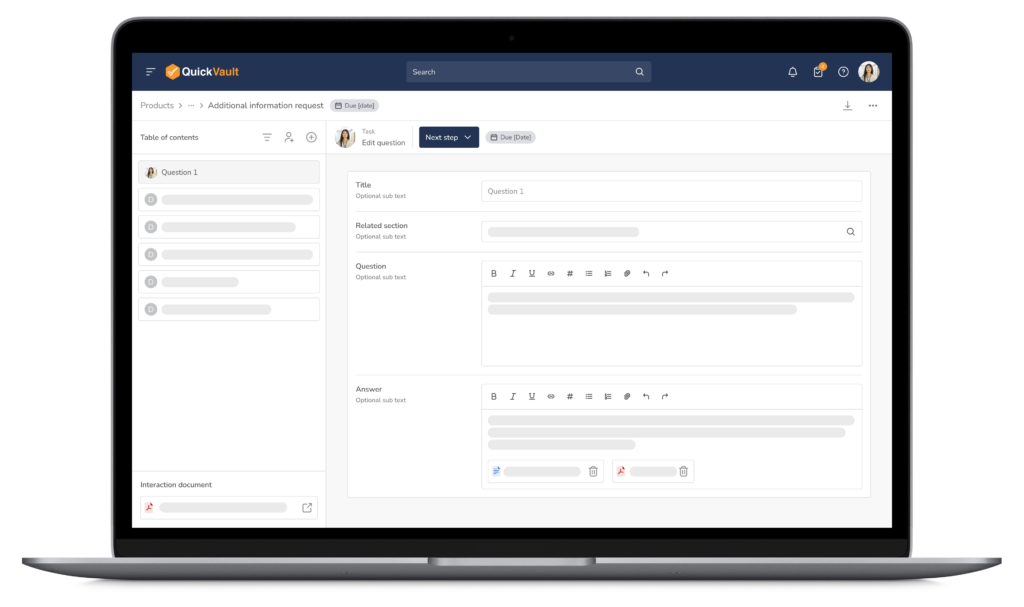
- Real-time submission collaboration
- Export the submission in an organized manner
- Ready to send to the FDA, your Notified Body, and other global authorities
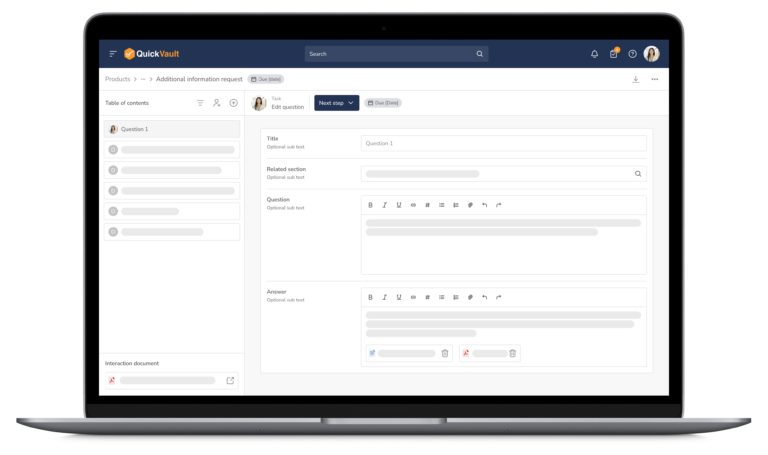
- Log and track questions and communication from regulatory authorities
- Assign follow-up actions to team members
- Maintain a complete audit trail
Frequently Asked Questions About Regulatory Submission Management
What types of submissions can you manage in QuickVault?
QuickVault allows you to manage any submission. From pre-submissions and IDEs to 510(k)s, PMAs, and CE marks, QuickVault gives MedTech teams a single, connected solution to prepare, track, and submit regulatory filings.
How does QuickVault support FDA eSTAR submissions?
QuickVault supports FDA submissions by integrating regulatory submission workflows directly into your eQMS platform. This helps MedTech teams prepare, track, and organize regulatory submissions that align with FDA expectations and quality system requirements, providing an eSTAR ready-to-upload package.
Can I track submissions across multiple markets?
Yes. QuickVault allows users to track any type of submission for any market in one system, giving regulatory teams a consolidated view of submission status, inter-agency interactions, and task progress across global regulatory authorities. Easily track FDA, EU MDR/IVDR, and other global submissions.
How does the regulatory module integrate with the rest of QuickVault?
QuickVault was built to provide a cross-functional and interconnected platform between documents, products, suppliers, equipment, submissions etc. Thus, as you go through Design & Development of a medical device, information and documents can be connected to a submission in parallel. Not only does this ensure that all information in the submission stays current with the latest document revisions, it also drastically reduces the time for submission creation.
Does QuickVault help you manage your interactions with regulatory agencies?
Yes, QuickVault makes it easy to manage your interactions with regulatory authorities, following submission. Assign responsibilities, track responses to individual questions, and respond to regulatory bodies with confidence and in a timely manner. QuickVault keeps all FDA and regulatory authority communications centralized, collaborative, and audit-ready.
Can multiple teams collaborate on the same submission?
Yes. QuickVault enables cross-functional collaboration between regulatory, quality, engineering, and clinical teams, with role-based access, task ownership, and full version control.
How long does it take to get started with the regulatory submissions module?
QuickVault is pre-configured for MedTech regulatory workflows, allowing teams to get up and running quickly without the need for an implementation and validation project. Most customers can begin preparing submissions days, not months.
Why MedTech Teams Choose QuickVault
One Connected Platform
Keep submissions, quality events, and design controls aligned for compliance visibility.
Built for MedTech Regulatory Readiness
Templates and workflows tailored for MedTech regulatory pathways.
Shorter Time to
Market
Reduce manual handoffs and accelerate your approval process.