When I transitioned into MedTech from a non-regulated industry, I initially viewed regulatory compliance as a burden. Stringent regulations, complex processes, and layers of documentation…it all felt like red tape getting in the way of what I wanted (and needed) to be doing: product development.
But over time, I came to realize something that surprised me; compliance, when done right, doesn’t hinder innovation. It enables it.
For MedTech startups especially, reframing compliance as a strategic driver rather than a box-checking obligation can unlock faster time to market, stronger submissions, and more scalable growth.
This blog explores a few ways that MedTech regulatory compliance makes companies more efficient, reduces time to market, and stronger once in commercial distribution.
How Smart Compliance Turns Regulatory Burden Into a Competitive Edge
This isn’t just a philosophy. A recent McKinsey & Company report found that medical device companies with “smart” quality systems can reduce time to market by nearly 30%. If you’re developing a Class II device on a typical 3-5 year timeline, that means launching your device to the market nearly a year earlier.
For early-stage companies, that’s not a luxury. That’s survival. Faster market entry means quicker revenue, more funding leverage, and a clearer path to scale. The key is understanding how compliance actually accelerates progress instead of slowing it down.
Why First-time MedTech Founders Struggle (and Why That’s Fixable)
The biggest mindset gap I see is with first-time founders in startups where MedTech regulations are new to everyone. In larger companies, like Boston Scientific and Medtronic, you’re surrounded by people with decades of regulatory knowledge and experience, which reduces the impact with employees who are new to the industry, and also accelerates knowledge transfer.
But in a three-person startup with first-time founders, compliance often feels abstract and therefore burdensome. While MedTech regulations aren’t rocket science, it becomes difficult just because you simply don’t know what you don’t know.
Thus, with regulatory compliance feeling like a barrier, the importance of recognizing the value of it becomes very important. The reality though is that many founders haven’t been exposed to the upside and benefit of adhering to the regulations.
They haven’t seen how a strong QMS and strong staff experience with operating under MedTech regulations (such as Design Controls) increases efficiency, reduces rework, prevents submission delays, and improves general crossfunctional alignment.
If more founders were aware that efficient MedTech regulatory compliance can literally shave a year off their go-to-market timeline, I think they’d embrace it faster. That’s why education and reframing are critical. So, if that’s you, I urge you to keep reading.
Design Controls: Project Management, with a Twist
One of the first regulations startups encounter is Design Controls, as outlined in FDA QMSR and ISO 13485. Design Controls includes the regulatory framework for how medical devices shall be developed, tested and documented.
Just like sound project management methodologies, such as those outlined in the PMBOK Guide by PMI, design controls are intended to support medical device teams in executing successful product development projects.
When followed properly, design controls help manufacturers bring products that are safe and effective for their intended use to market, reducing the risk of field failures that could harm patients or users.
That kind of success includes two critical elements: thorough upfront planning and the involvement of a crossfunctional team that collaborates from development start to commercial launch.
Unlike traditional project management, upfront planning isn’t just about timelines, stakeholders and task lists. It’s about aligning your entire team around regulatory expectations, risk-informed decisions, and evidence-based iteration. In MedTech, your project plan should also serve as your compliance framework.
High performing MedTech teams use design controls to:
- Clarify requirements across all stakeholders, including engineering, manufacturing, medical, quality, regulatory, marketing, sales, etc.
- Ensure thorough risk assessments have been conducted early on, covering the design of the device, the manufacturing, its use, and disposal.
- Align early on intended use and user needs
- Minimize gaps that trigger late-stage rework or failed submissions
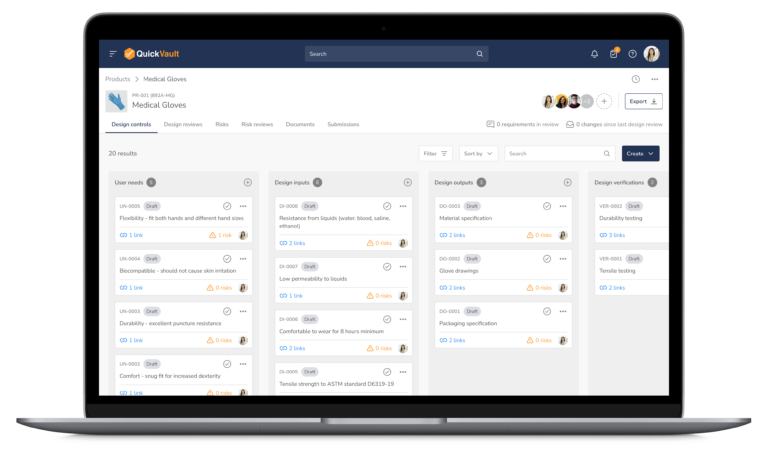
At Veeva, we built our QuickVault product to support startup teams with staying in compliance while being efficient, even though they may lack previous regulatory experience and knowledge.
The workflows within QuickVault support the “twist on project management” by providing full Design Control and Risk Management automation. With the functionality in QuickVault, all Design Controls, including Design Reviews, can be done on the platform.
This ensures that teams stay aligned, move faster, and set themselves up for regulatory submission success without managing compliance in various disconnected documents and systems.
Implementing Design Controls
When it comes to designing and developing a medical device in compliance with regulations, design controls need to be at the top of your list, as this is a key component for ensuring regulatory submission success. So when does a company need to implement design controls?
While the regulations do not explicitly state when a medical device manufacturer has to conform to design controls, at a minimum, you must adhere to these regulations when formal development begins (i.e., at the time your device is being tested to generate data for demonstrating device safety and effectiveness).
While this is the latest stage to begin following design controls, some companies elect to start earlier in the R&D process during the Research or Feasibility phase (when early prototypes are developed) of the project.
Regardless of timing, one thing is clear: successful teams use design controls as a planning and decision-making framework. They don’t just document what they’ve done after the fact.
Design Control Documentation: Efficiency & Speed
For fast-moving development teams, progress is made on a daily basis. Most of that progress results in various documents such as drawings, protocols, test reports etc.
The amount of documents and data, and their interconnectivity, can quickly grow to a point where it becomes very difficult to manage and have transparency into. The traditional way of using spreadsheets and shared drives break down fast.
If you don’t have a solution that automates your design control workflows by connecting your user needs to design inputs, verifications, risk controls, and validations, etc., you’re going to spend a significant amount of time looking for documents and data to ensure completeness and will be constantly second-guessing your work.
A system with built-in design control automation will:
- Allow for full transparency of design control items and their interconnectivity
- Provide a single source of truth for product related files like the Design and Development File and Medical Device File
- Automatically version control documents and track changes
- Allow you to link artifacts across the product lifecycle
- Show you exactly what impact a change has downstream
At QuickVault, we designed our platform to make this easy for MedTech startups. You shouldn’t need an enterprise team or six months of configuration to achieve efficiency in your product development projects.
Our MedTech-specific workflows are built to support design controls, risk management, and document control from day one—without overcomplicating your process. See QuickVault in action by booking your personalized demo today ➝
Innovation Requires More Than Engineers: The Importance of a Cross-functional Team
Strong engineering alone isn’t enough to launch a successful medical device. Efficient and successful medical device development and commercialization happens when teams are cross-functional by design.
It’s not enough for MedTech design engineers to operate in a silo, and hand over to other stakeholders within the organization at the last minute. The best teams bring in quality, regulatory, medical, manufacturing/operations, and even sales and marketing early in the Design & Development planning process.
However, that doesn’t mean every stakeholder should be in every meeting. It means your design and development plan should clearly lay out who needs to be involved and when. This prevents gaps, miscommunication, and missed business opportunities.
Culture & Capability: Regulatory Compliance is a Team Sport
Regulatory compliance isn’t just about submitting the right paperwork on time. It’s about building a culture of quality and a team that knows how to execute it. Training matters. Internal education matters. And leadership buy-in matters.
At QuickVault, we’re not just focused on software. We’re invested in helping MedTech teams grow into mature, high-performing, efficient, organizations. That means giving them the tools and the mindset to succeed.
Putting Smart Compliance into Practice
I understand the skepticism around compliance. I’ve lived it. But I’ve also seen what happens when teams build the right systems and mindsets from the start.
They move faster. They build better products. They create commercially successful companies, while staying audit-ready without the scramble. And they create space to focus on what really matters: delivering safe, effective devices to patients.
That’s exactly what we’re enabling at QuickVault. If you want to see how our purpose-built eQMS can help your team turn smart compliance into a competitive advantage, we’d be happy to show you. Book your personalized demo and let’s walk through it together.