For early-stage MedTech startups, building a medical device is only half the battle. Bringing it to market, and keeping it there, means meeting a complex set of quality and regulatory expectations. That’s where a Quality Management System comes in.
But here’s where it gets confusing. What exactly is a QMS? Is it a binder of SOPs, a spreadsheet on a shared drive, or the cloud-based software your consultant keeps mentioning? And what’s the difference between that and an electronic QMS (eQMS)?
If you’ve asked these questions, you’re not alone, and you’re asking them at the right time. Understanding the distinction between a QMS and an eQMS can save your team time, reduce compliance risk, and help you scale faster.
Let’s break it down.
A QMS Is the Framework Behind Your Quality Processes
A QMS isn’t a tool. It’s a structured set of procedures, policies, and processes that ensure your product meets both customer needs and regulatory requirements. Think of it as the foundation of how your company operates when it comes to quality.
Regulations and standards like FDA’s 21 CFR Part 820 and ISO 13485:2016 require most medical device companies to establish and maintain a QMS. But they don’t dictate how that system should be managed, only that it must exist and function effectively.
For startups, this often begins with a lightweight, “paper-based” QMS. You might create controlled documents in Word, store them in Google Drive, and manage version control manually. CAPAs might live in spreadsheets. Training records might be signed PDFs saved on someone’s desktop.
It’s scrappy, but in the early stages, it works. And for a while, it might even feel efficient. If you’re just beginning, this step-by-step guide to QMS setup for MedTech startups can help you structure the essentials.
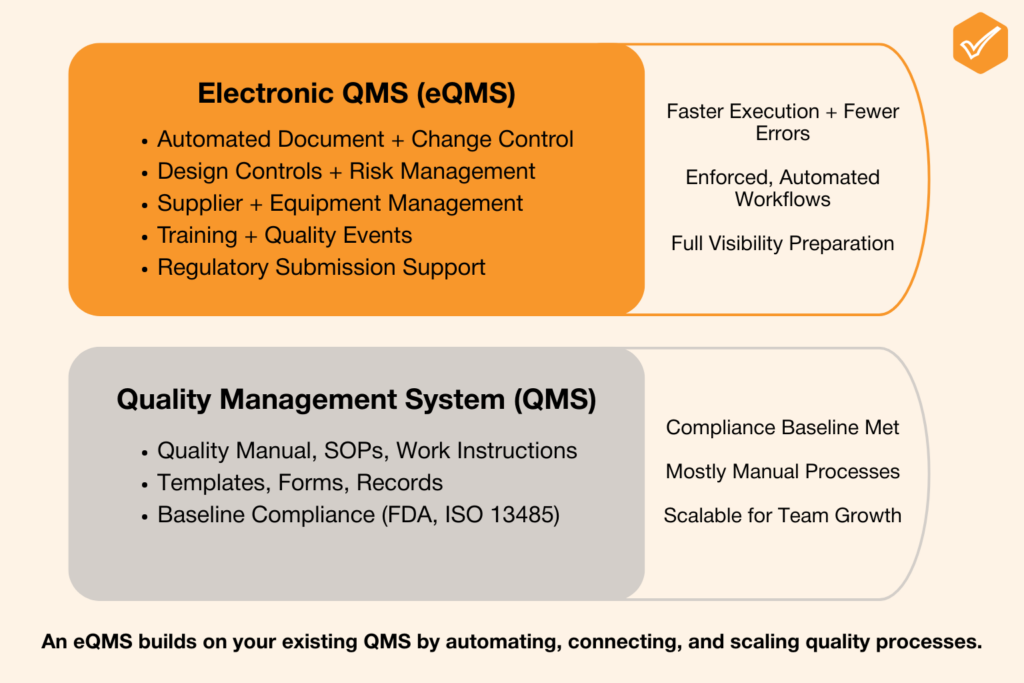
An eQMS Is the Digital Infrastructure That Supports and Automates the QMS Framework
Whereas a QMS is the system itself, an eQMS is the software you use to build, enforce, and scale that system. A good eQMS brings structure and automation to core quality processes like:
- Document control
- Design controls and traceability
- Training and competency tracking
- CAPA management
- Audit preparation and reporting
- Risk management
- Complaint handling
- Supplier qualification
An eQMS doesn’t just digitize your files, it creates a single source of truth for your entire quality system. More importantly, it enables better collaboration, enforces compliance workflows, and makes it significantly easier to pass audits and inspections.
FDA doesn’t require an eQMS, but it does expect your QMS to be complete, consistent, and inspection-ready. And that’s where manual, “paper-based” systems start to break down.
The diagram below shows how an eQMS builds on the foundation of a traditional QMS, layering in automation, traceability, and scalability to help MedTech teams meet growing regulatory demands.
Why This Matters for MedTech Startups
Most MedTech startups don’t implement an eQMS on day one, and they shouldn’t. You’re moving fast, wearing multiple hats, and trying to prove feasibility. But as your device moves closer to submission, clinical use, or commercialization, the cracks in a manual QMS start to show.
We’ve seen it firsthand:
- One company spent hours navigating shared folders and naming conventions just to find the right document version for a regulatory milestone. (Read their story)
- Another company lost months scrambling to trace design inputs to user needs because their spreadsheets weren’t properly updated.
- One team missed a critical CAPA deadline because it was buried in an old email thread with no automated reminders.
These issues aren’t just administrative, they can delay approvals, trigger nonconformances, damage your reputation with regulators and partners, and even devalue your company in the eyes of potential investors—ultimately impacting your exit opportunities and valuation.
For a deeper dive into timing considerations, read our article When Is the Right Time for a MedTech Startup to Implement a QMS?
QMS vs. eQMS: A Side-by-Side Look
Capability
Paper-Based QMS
eQMS Platform
Compliance
Possible (with effort)
Built-in workflows
Version Control
Manual
Automated and enforced
Audit Readiness
Time-intensive
Instant reports/logs
Training Records
Spreadsheets/PDFs
Linked to role and SOPs
Change Management
Email-dependent
Tracked and documented
Team Collaboration
Fragmented
Centralized and scalable
Risk of Human Error
High
Lower
From a compliance standpoint, a QMS is required for Class II and Class III medical devices, but FDA doesn’t mandate how that system must be managed. While you’re not required to use an eQMS, relying on manual systems can quickly become a liability as your team grows and regulatory demands increase.
If you’re comparing solutions, our MedTech eQMS Selection Guide can help.
Signs It’s Time to Move from a Paper-Based QMS to an eQMS
If your team is still relying on shared drives, spreadsheets, and disconnected tools to manage your QMS, chances are you’ve already felt the friction. Here are common indicators your paper-based system is holding you back:
- You’re preparing a 510(k) submission or ISO 13485 audit: Suddenly every document needs tracking. Version control becomes a scavenger hunt.
- Your team is growing: More people, more document chaos, more training gaps.
- You’re expanding your product line or entering new markets: Complexity adds exponentially more documentation.
- You’re spending more time on admin than product development: Manual tasks like chasing signatures are stealing your time.
- You’re anxious about inspection readiness: If an auditor walked in tomorrow, would you be prepared?
These are more than growing pains, they’re warning signs. The longer you wait, the more tangled your processes become.
That’s where QuickVault comes in.
QuickVault by Veeva is purpose-built for MedTech startups that need to move fast without compromising compliance. Its easy-to-use eQMS helps your team get out of spreadsheet chaos and into a centralized, audit-ready system that scales with you.
With QuickVault, you can:
- Automatically track document versions and approvals
- Connect design controls to user needs, risks, and requirements
- Streamline training and keep records up to date
- Build in traceability without manually linking artifacts
- Generate audit-ready reports and submission documentation with just a few clicks
You’re already doing the work to stay compliant, QuickVault removes the stress, making it easier and faster than ever. For a practical overview, watch our QMS Implementation on-demand webinar.
Beyond Compliance: Building a Culture of Quality
Implementing an eQMS isn’t just about passing audits, it’s about embedding quality into your company’s DNA. That mindset matters. It impacts how your team works, how investors perceive you, and how confidently you engage with regulators.
Quality isn’t just mandatory, it’s one of your best strategic assets. Want proof? Here’s how compliance can drive innovation when done right.
Turn Your QMS Into a Strategic Asset with an eQMS
Here’s the bottom line:
A QMS is the foundation that ensures quality and compliance. But without proper tools, it can become a bottleneck. An eQMS transforms your QMS from fragile to future-proof, streamlining compliance and empowering your growth.
QuickVault by Veeva helps you get ahead with an eQMS built specifically for MedTech startups. It’s intuitive, scalable, and audit-ready, so your QMS becomes your competitive edge.
Try QuickVault now to see how our solution can help you simplify compliance and grow with confidence.