Medical Device Equipment Management Software
Equipment Management Built for MedTech
Streamline MedTech operations with one connected platform for equipment management. Control equipment, calibration, maintenance, and validation all inside your QMS.
Smarter Equipment Management for MedTech
QuickVault Equipment Management offers a built-in Computerized Maintenance Management System (CMMS) solution for medical device companies, fully integrated into the eQMS platform.
MedTech teams can manage equipment lifecycle, calibration, preventive and corrective maintenance, qualification, and process validation, all while maintaining compliance with global regulations and standards.
Why Equipment Management is a
Regulatory Priority in MedTech
Equipment plays a critical role across the medical device lifecycle, from early development through commercial manufacturing. At every stage, equipment must be properly specified, installed, documented, and validated.
Because manufacturing equipment directly impacts product safety and performance, regulators require it to be controlled. This includes calibration, maintenance, qualification, and, where applicable, validation to ensure devices consistently meet regulatory and quality requirements.
The Challenges of Equipment Management
for MedTech Teams
Most MedTech companies start with:
- A master spreadsheet of equipment used for R&D, manufacturing, and tests
- Manual tracking of calibration and maintenance dates
- Manual tracking to Quality Events related to equipment
- Calendar reminders in Outlook or Google Calendar for scheduled calibration and maintenance
- Paper or PDF forms for approvals
This creates serious problems:
- Missed calibrations or maintenance
- Manual, error-prone tracking
- No visibility into what equipment was used for which product
- Time-consuming investigations during quality events
- Little to no trending of equipment-related NCRs, CAPAs, or Complaints
QuickVault eliminates these gaps by embedding CMMS functionality directly into your QMS.
Core Equipment Management CMMS Capabilities
Complete Visibility Across
Equipment Operations
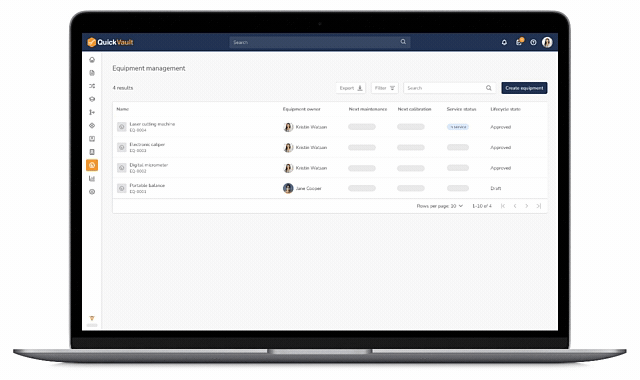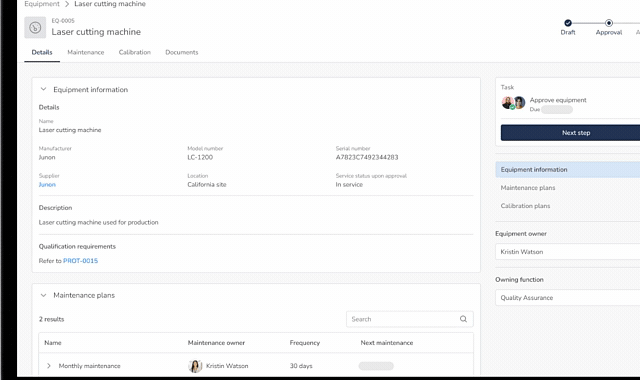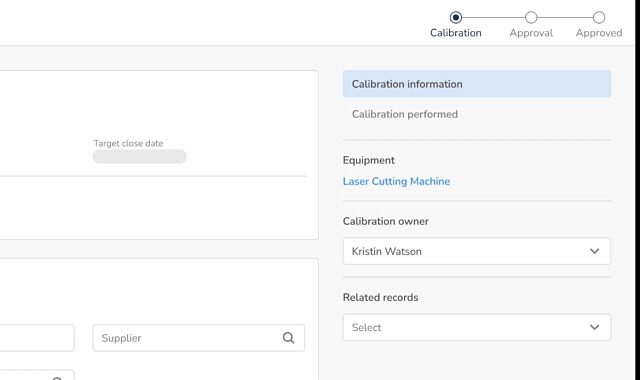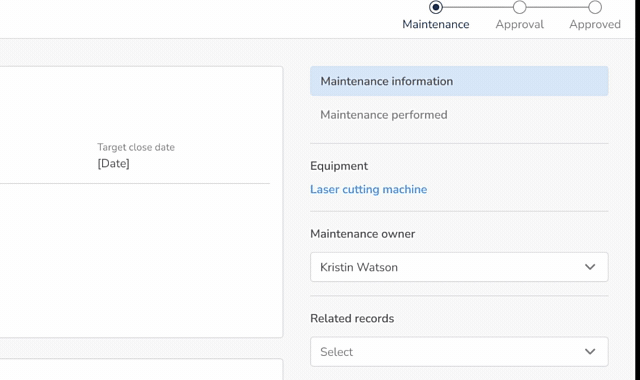
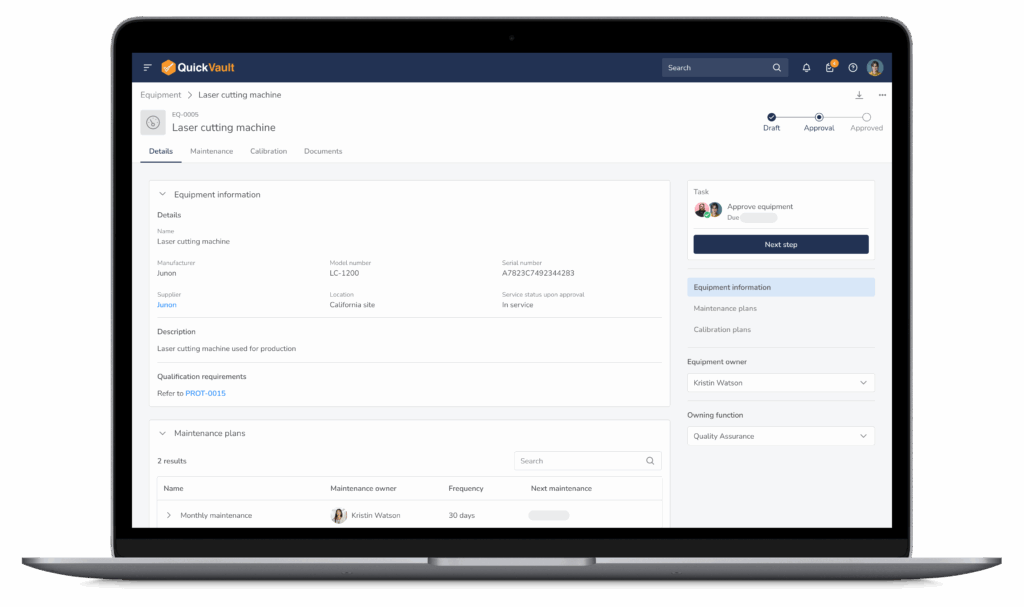
QuickVault gives MedTech teams one system to manage documents, products, equipment, suppliers, and quality events. No spreadsheets. No disconnected calendars. Just audit-ready equipment control from design through commercial manufacturing.
Equipment Lifecycle Control
- Add and manage all equipment in one system
- Capture manufacturer, model, serial number, purchase and supplier information
- Take equipment in and out of service during installation, breakdown and quality event investigations
- Connect equipment to qualification activities such as ranging studies, IQs, OQs, PQs, etc.
Calibration Management (21 CFR 820.72)
- Define calibration requirements based on product and process risk
- Schedule calibration using built-in scheduling functionality
- Assign user responsibilities for execution of calibration
- Maintain complete calibration records and evidence
- Ensure measurement equipment provides accurate, reliable results
- Connect out-of-calibration equipment to Non-conformances
Preventive Maintenance (PM)
- Define PM requirements per manufacturer instructions
- Schedule recurring maintenance activities
- Assign maintenance execution ownership and due dates
- Maintain documented evidence of work performed
- Reduce unexpected downtime and failures
- Connect issues found during PM to Non-conformances and initiate Corrective Maintenance as needed
Corrective Maintenance (CM)
- Manage unplanned maintenance and repairs
- Automatically link CM to Quality Events (NCRs, CAPAs, Complaints)
- Bound investigations to affected equipment and impacted parts, assemblies or products
- Support root cause analysis and corrective actions
Connectivity in One Place
- Connect equipment information to documents, products, suppliers, and quality events across the platform.
- Organize and link documents, design controls, risks, and quality events directly to equipment, maintenance, and calibration records.
Frequently Asked Questions About Equipment Management
What is medical device equipment management software?
Medical device equipment management software helps manufacturers control, calibrate, maintain, qualify, and validate equipment used in design, testing, and manufacturing. It ensures compliance with FDA regulations, ISO 13485:2016, and EU MDR by maintaining traceable, audit-ready records for equipment activities.
How does QuickVault support FDA 21 CFR 820 equipment requirements?
QuickVault supports FDA equipment requirements under 21 CFR 820.72 and 21 CFR 820.75 by enabling calibration scheduling, preventive and corrective maintenance tracking, equipment qualification, and process validation documentation. All records are centralized and traceable for inspections and audits.
What is the difference between preventive and corrective maintenance in medical devices?
Preventive maintenance (PM) includes planned activities performed to keep equipment functioning properly, such as routine inspections or part replacements. Corrective maintenance (CM) is unplanned work performed to restore equipment after a failure or malfunction. In QuickVault, corrective maintenance is linked to Quality Events such as NCRs or CAPAs.
Can QuickVault manage calibration for inspection, measuring, and test equipment?
Yes. QuickVault allows teams to define calibration requirements, schedule calibration activities, assign responsibility, and maintain calibration evidence in accordance with FDA 21 CFR 820.72 and ISO 13485:2016 requirements.
Does QuickVault support equipment qualification and IQ, OQ, PQ validation?
Yes. QuickVault supports equipment qualification and process validation by linking IQ, OQ, and PQ protocols, reports, and Master Validation Plans directly to equipment. This ensures equipment is qualified and validated before use in design verification, validation, or manufacturing.
How does equipment management support design verification and validation (DV&V)?
By linking equipment to products and validation documentation, QuickVault provides visibility into what equipment was used during design verification and validation activities. This supports traceability, risk management, and impact analysis when design or process changes occur.
How does QuickVault connect equipment to quality events?
Equipment can be linked directly to Quality Events such as NCRs, CAPAs, and Complaints. This allows teams to assess equipment as a potential root cause, track recurring issues, trend data over time, and take proactive corrective action.
What are the benefits of using QuickVault Equipment Management?
QuickVault Equipment Management is purpose-built for MedTech teams. It is the only eQMS with built-in CMMS, automated scheduling & accountability, full traceability across products, documents, and quality events, and is specifically designed for FDA, iSO13485, and EU MDR audits.
Book your personalized walkthrough of QuickVault’s Equipment Management Functionality to discover the benefits for MedTech teams.
Why MedTech Teams Choose QuickVault
One Connected Platform
Track, approve, and manage equipment and assets in one centralized platform.
Built for FDA Readiness
Templates and workflows tailored for MedTech regulatory pathways.
Faster Time to
Market
Reduce manual handoffs and accelerate your approval process.